According to EPA standards, a disinfectant should be able to kill 99.9 percent of disease-causing bacteria and viruses. Which of the following formed? Which of the following formed? Write balanced net ionic equations for the reactions, if any, that occur between the following: Metathesis Reactions: Lead nitrate + sodium sulfide H. Q:CO2 and SiO2 have similar formulae but very different melting points, why? Pb2+(aq) + 2NO3(aq) + 2H+(aq) + SO42(aq) PbSO4(s) + 2H+(aq) + 2NO3(aq). I Put the fine, pulpy grated cabbage into a large bowl or pot. Here are a couple fun things you can do with these two substances. Soak steel wool in vinegar and watch what happens as the iron in the steel begins to react with the oxygen around it. From here on out I will write the Just use full-strength lemon juice to write an invisible message on paper and let the message dry. Woman gets back at husband who got unflattering tattoo of her, Stacey Solomon praised for refreshing post of her normal body, Vet issues emotional advice to pet-owners putting their animals down, Millie Radford accuses her mum-of-22 of making money out of grandkids, Tom Daley and husband Dustin Lance Black announce theyve had another baby, Horrified shopper demands Sainsburys rename wildly inappropriate and sexist steak, Mum-of-22 Sue Radford responds after daughter accuses her of making money out of grandkids, Woman explains the difference between bio and non-bio washing powder and people are genuinely thankful, Mums are just discovering hidden feature on nappies that helps to avoid blowouts, People left gobsmacked after woman discovers 'mind-blowing' dishwasher hack, Woman shares 'life saving' hack for opening tight glass jars. What decimal power does the abbreviation n represent? Vinegar
Guaranteed to get rid of the mice, she said. What is the effect of household ammonia on dry litmus? What is the effect of H2SO4 on Epsom salts? -soluable in water. Materials
Calculate the HDI for each molecular form Sodium hypochlorite, the active ingredient in chlorine bleach, is routinely used in the laboratory to decontaminate surfaces and equipment or deactivate biological materials by inactivating vegetative bacteria, fungi, lipid and non-lipid viruses, and other liquid specimens. While a mixture with bicarb is considered safe, some group members used the post as an opportunity to flag the very real dangers of combining potent chemicals in cleaning products. What other characteristics do many gymnosperms have? WebDo not mix vinegar or acidic liquids with bleach, as the combination can be dangerous. Which of the following formed? -When mixed with mineral oil, the solution turned purple. A:The reaction is SOH, Q:Draw the structure of all the alkyl halides and nucleophiles/bases used in the above Its pKb can be calculated as: Metathesis Reactions: Potassium chloride + sodium nitrate Metathesis Reactions: Cadmium chloride + sodium hydroxide Which of the following formed? Laboratories should clean up small spills themselves, provided they are knowledgeable of the hazards and have the proper PPE. Metathesis Reactions: Hydrochloric acid + sodium hydroxide Is this substance a liquid or solid at room temperature? Silicone Oil 200/50 cst NiCl2(aq) + Na2CO3(aq) NiCO3(s) + 2NaCl(aq). If you mix acidic vinegar with basic baking soda and stow them away in a closed container, the mixture can be quite explosiveliterally. H2 (g) + Br2 (g) 2 HBr (g) CA 70 Rust out Iron Out Inc. 71 Scrub Free bathroom cleaner / soap scum remover Church & Dwight Canada Corp. Did the solution color indicate that the lime or lemon juice and vinegar were acidic (had a lower pH) and that the bleach was basic (with a higher pH)? This includes acidic drain cleaners, rust removers and even vinegar. What is the mass in kilograms of 690 mL of a substance that has a density of 0.860 g/mL? Metathesis Reactions: Lead nitrate + sodium sulfide Metathesis Reactions: Lead nitrate + sulfuric acid Prepare a fresh working dilution of sodium hypochlorite weekly and indicate the preparation date on the bottle. According to the Virginia Department of Health, acetic acid has the chemical formula CH3COOH. Which of the following reactions are metathesis reactions? Explanation: Sodium hypochlorite is a strong electrolyte that ionizes in sodium cation and hypochlorite anion. What is the net ionic equation for this reaction? What is left at the end of the reaction, and after all the Cl2 gas has been purged from the solution? IV Copyright Complaints, Spill Response Standard Operating Procedure (SOP), Brethericks handbook of reactive chemical hazards, https://www.ncbi.nlm.nih.gov/books/NBK537213/, https://www.atsdr.cdc.gov/phs/phs.asp?id=51&tid=16. 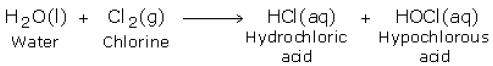
 For example, red cabbages contain an indicator pigment molecule called flavin, which is a type of molecule called an anthocyanin. Will CH3CH2CH2NH3 act as a Bronsted-Lowry acid or base when reacting with water? Each of these products can easily clean a mess on its own, but together, they lose their ability to effectively clean your home. Many who have poured bleach into a toilet bowl following an unsuccessful attempt to remove stains with a commercial cleaner have suffered permanent lung damage and some have died. Determine the boiling point of water at 695 mmHg . You die of stupidity Misuse of chemical names leads to nasty consequences to people who think they know what they are doing but do not. Sodium hypo The woman from Melbourne, said she was left feeling frustrated after trying 'every product' on the walls of her bathroom in her rental property. Why is it necessary to calibrate a thermometer and volumetric glassware? O A. IV Which of the following are not water-soluble: Write equation for the decomposition of H2CO3, Write equation for the decomposition of H2SO3. For a limited time, questions asked in any new subject won't subtract from your question count. 0000043649 00000 n
This is the best answer based on feedback and ratings. -CH-SOH That would then be a solution of acetic acid, sodium acetate, and sodium chlorate. Fischer projection shown below. another added. A 136 mg sample was placed on a watch glass that has a mass of 7.482 g . 2Na+(aq) + CO32(aq) + 2H+(aq) + SO42(aq) H2O(l) + CO2(g) + 2Na+(aq) + SO42(aq). Bleach is an oxidizer and corrosive. Pour vinegar on to the steel wool and allow it to soak in the vinegar for around one minute. Metathesis Reactions: Ammonium chloride + sodium hydroxide Which of the following formed? Thanks for reading Scientific American. CH3 Ammonia is a chemical compound with the formula NH3. Is it safe to mix hydrogen peroxide and vinegar? Ill say Martin Pitt answered it pretty well, and just expand on that a bit. Since vinegar is 5% a To make sure you never miss out on your favourite NEW stories, we're happy to send you some reminders, Click 'OK' then 'Allow' to enable notifications, .css-o3g03s{color:black;}Published15:03,03 April 2023 BST| Last updated14:51,03 April 2023 BST, Featured Image Credit: Facebook/Mums Who Cook, Clean and Organise. What color did the solution become? What decimal power does the abbreviation k represent? Hydroxypropyl Methylcellulose. This took me only a few minutes and not a lot of effort - I couldn't believe my eyes.". "Non-porous' surfaces such as hard plastics should be relatively easier to clean. What ingredients are in bleach? Ammonia and Vinegar Chemical Equation. 4 (Chemical Connections 19F) Why do Lactomer stitches dissolve within 2 to 3 weeks following surgery? neutral, acidic or basic. Goggles or other protective eyewear
Why should you never determine the mass of a hot object? What is the complete ionic equation for this reaction? H2SO4 would react with both to form gasses, so the presence of HCl would mask Na2CO3. Take care when combining bleach with other cleaning products. What decimal power does the abbreviation m represent? RaSO4 Vinegar only works against some germs, like E. coli and Salmonella. equivalence point on, A:The question is based on the titrations. 'Well I know what I'm doing while the kids are at school," another said. Draw a flow sheet to show how you would separate the components of a mixture containing an acid substance, toluic acid, a basic substance, p-bromoaniline, and anthracene, a neutral substance. If the activation energy for the, A:Enthalpy change = Ef - Eb Pour vinegar on to the steel wool and allow it to soak in the vinegar for around one minute. Thanks for reading Scientific American. After using water as a base, a typical bottle of bleach contains: ( 2) If an average man is 5 ft 10 in. How basic or acidic a solution is depends on the amount of hydrogen ions in it. Rusting (or oxidation) is a chemical reaction between iron and oxygen, this chemical reaction creates heat energy which increases the temperature inside the beaker. Bleach-based cleaning product
Disodium Lauryl Sulfosuccinate Write the chemical equation for the reaction between MgCl2 and the soap prepared. What is the molecular equation for this reaction? What is the net ionic equation for this reaction? True or False: Innocuous common household chemicals, like bleach and ammonia, can be combined without producing severe explosions or other hazardous reactions. K2CO3(aq) and Cu(NO3)2(aq), Write balanced net ionic equations for the reactions, if any, that occur between the following: Because it's a weak acid they are adding, that does not react with sodiumhypochlorite apart from the simple acid base reaction. If you were to mix This fun science experiment for kids is great for learning about chemical reactions. Metathesis Reactions: Copper(II) sulfate + sodium carbonate What is the complete ionic equation for this reaction? Recommended working dilution: 5250 ppm (1:10 dilution of household bleach of 5.25% sodium hypochlorite), Recommended for floors, spills (inactivating liquid specimens), bench tops and contaminated clothing. The experts from consumer company Choice said the first thing you need to do is assess the surface the fungi has attached itself to. Find answers to questions asked by students like you. ||| Metathesis Reactions: Cadmium chloride + sodium hydroxide Volume of NaOH solution = 9.20 mL = 0.0092 L, Q:The formation of glucose from water and carbon dioxide is an extremely important reaction for life, A:Recallthegivenreaction,H2Og+CO2gC6H12O6s+O2Ata, Q:At 500 K, hydrogen and iodine can form hydrogen iodide in the gas-phase reaction: Which of these substances is least soluble on a gram-per-100 mL basis? rearrangement is not true? What is the molecular equation for this reaction?
(D) SF6, A:A dipole moment is a unit used to describe how far apart two electrical charges are from one, Q:An elementary process has an activation energy of 40 kJ/mol. But the smell grows even more acrid once you add vinegar because the combination releases chlorine and chloramine vapors, which can cause a chemical burn. When recording your observations with K2CrO4K2CrO4, which of the following occured? Find the mean volume of the samples. H+(aq) + Cl(aq) + Na+(aq) + OH(aq) H2O(l) + Na+(aq) + Cl(aq). Metathesis Reactions: Ammonium chloride + sodium hydroxide Which of the following formed? 0000044024 00000 n
The number 3.27 10^-3 cm has ________ significant figures. Metathesis Reactions: Cadmium chloride + sodium sulfide What is the complete ionic equation for this reaction? -The water turned brown. p-Phenylenediamine (also named 1,4-diaminobenzene) dyes hair black. Write the chemical equation for the reaction of washing soda, Na2CO3 , with HCl. It converts aldehydes and ketones directly into alcohols. Q:Question 4: III Cover the beaker with paper or a lid to keep the Which of the following are strong electrolytes: Which of the following are weak electrolytes? Pouring vinegar over the pennies helps break up this copper oxide and expose the pure copper on the penny. Given the reaction below: 2. Lactic Acid 90% The solubility is 2.1x10-4 g/100 mL at 20C Get access to millions of step-by-step textbook and homework solutions, Send experts your homework questions or start a chat with a tutor, Check for plagiarism and create citations in seconds, Get instant explanations to difficult math equations, Types of Polymers on the basis of Method of Preparation. Lemon or lime juice
The acrid fumes of chlorine can destroy lung tissue, cause the lungs to fill with water and in a sense cause death by drowning. Q:Equimolar amounts of H2(g) and Br2(g) are injected into an evacuated, rigid container, where they, A:Given chemical reaction Chlorine gas was of course used for this purpose in World War I. Q:23. WebSodium hypochlorite, commonly known in a dilute solution as (chlorine) bleach, is an inorganic chemical compound with the formula NaOCl (or NaClO), [3] comprising a What is the net ionic equation for this reaction? HSO4 It is a colorless liquid with a corrosive pungent vinegar-like odor with a sour taste. Metathesis Reactions: Ammonium chloride + sodium hydroxide II NiCl2(aq) + 2AgNO3(aq) 2AgCl(s) + Ni(NO3)2(aq). 0000007898 00000 n
DMDM HYDANTOIN. Bases
0000023453 00000 n
Besides chlorine gas after mixing these two chemicals what liquid byproduct is left? WebAnswer (1 of 82): Is it safe to mix hydrogen peroxide and vinegar? Which of the following statements about carbocation long? c. urea KCl will dissolve in water; PbCl2 will not dissolve in water. CH3 "Acids, Bases, and the pH Scale" from Science Buddies
Low levels of exposure may result in eye and oral mucous membrane irritation, dizziness, and nausea while exposure to high levels may be fatal. What is the molecular equation for this reaction? CuSO4(aq) + Na2CO3(aq) CuCO3(s) + Na2SO4(aq). View this solution and millions of others when you join today! What is a cone? Just like combining bleach with vinegar is a bad idea, so is mixing bleach with rubbing alcohol. Store below eye level with compatible chemicals (Stanford Compatible Storage Group E). Treat one portion with concentrated H2SO4H2SO4 and look for violet vapors to indicate the presence of iodide Sketch a plot of pH vs volume of added NaOH for titrating vinegar. Which of the following formed? 0000008146 00000 n
Captioning some pretty impressive before and after photos on the Facebook group Mums Who Cook, Clean and Organise, she said: "Mums! Tween 80 Permanently. For health emergencies, call 911 (9-911 from a campus phone). KCl(aq) + NaNO3(aq) KNO3(aq) + NaCl(aq). Metathesis Reactions: Copper(II) sulfate + barium chloride 1. Add NaOH and look for a color change with red litmus paper, Mix with H2SO4 to release CO2 gas, then detect the CO2 with Ba(OH)2, Add H2SO4 and look for a color change with blue litmus paper, Look for white precipitate to form when mixed with BaCl2, Look for a pale yellow precipitate when treated with AgNO3, Look for a yellow precipitate when mixed with I- ions. Metathesis Reactions: Nickel chloride + sodium carbonate But she neglected to say that it could getrid of the human inhabitants as well. Cd2+(aq) + 2Cl(aq) + 2Na+(aq) + S2(aq) CdS(s) + 2Na+(aq) + 2Cl(aq). Which different sources of pigment produce the best indicators? Enjoy our range of fun science experiments for kids that feature awesome hands-on projects and activities that help bring the exciting world of science to life. Most toilet bowl cleaners contain sodium hydrogen sulfate, an acid which will quickly liberate chlorine from bleach. NH4Cl(aq) + NaOH(aq) NH3(g) + NaCl(aq) + H2O(l). The color of the solution will change depending on its pH: Red color indicates the pH is 2; Purple indicates pH 4; Violet indicates pH 6; Blue indicates pH 8; Blue-green indicates pH 10; Greenish-yellow indicates pH 12. C6H602 First week only $4.99! -exothermic (heat-producing) Which of the following is/are listed as active ingredients? Mixing with bleach releases oxygen in a closed system (e.g., piping, equipment) which can lead to pressure build-up and rupture. Consequently, the color an anthocyanin solution turns can be used to determine a solution's pHa measure of how basic or acidic a solution is. 3CuSO4(aq) + 2Na3PO4(aq) Cu3 (PO4)2(s) + 3Na2SO4(aq). Chlorine compounds have some effect in inactivating bacterial spores: Refer to the Biosafety Manual for additional information and guidance on selecting appropriate disinfectants, or for appropriate bleach use with prions and prion-like proteins. Zinc Oxide Handling it with caution, add drops of the bleach cleaning product until you see the solution change color. Place a strainer over another large bowl or pot and pour the cabbage mixture through the strainer to remove the cabbage pulp. The HOCl is called hypochlorous acid. O Give the functions of the following ingredients, then name a branded/commercial skin or hair care product where the said material is used. between adjacent, Q:Calculate the Grxn using the following information. Step 3: Cover the plants around your home with a plastic sheet to prevent the bleach from damaging them. A solution with a pH between 5 and 7 is neutral, 8 or higher is a base, and 4 or lower is an acid. What role do cones play in gymnosperm reproduction? WebReaction + Balanced Equation. This should take at least half an hour. OH You'll get a detailed solution from a subject matter expert that helps you learn core concepts. If you do not want to grate the entire cabbage, grating half of a cabbage should be enough. Metathesis Reactions: Cadmium chloride + sodium sulfide In fact, together theyre quite potent. Metathesis Reactions: Sodium carbonate + sulfuric acid The toxicity of ammonia is dependent on the source of the ammonia, whether it is an animal or plant, and its concentration. -Distillation removes most chemicals present in tap water that could give us false results. "When mould grows, it develops hyphae, or roots, which grow into the grout or silicone. Dont take chances when it comes to the safe use of cleaning products, says Brian Sansoni from the American Cleaning Institute. Part complete 1) washing soda and vinegar (sodium carbonate and HC2H3O2) 2) Vitamin C and ammonia (H2C6H6O6 and NH3) Best Answer. Metathesis Reactions: Lead nitrate + sodium sulfide Metathesis Reactions: Nickel chloride + silver nitrate "Just bought Domestos! How could you show the presence of both carbonate and chloride in this mixture? -gases are produced. Mould growing on grout and tiles can also give off toxic spores and vapours which can lead to allergic reactions, asthma and flu-like symptoms. Grate a small red cabbage. H. Yellow precipitate is formed. CH3 Write a balanced equation for the reaction: HCl(aq)+Na2CO3(s)? WebAnswer (1 of 4): I am waiting for you to try it and find out. spectroscopy. d. ammonium hydroxide. What mass of Bleach will corrode metal including metal wastewater pipes. WebThe following is a step-to-step procedure. Source: Handbook of Chemistry and Physics. CdCl2(aq) + 2NaOH(aq) 2NaCl(aq) + Cd(OH)2(s). This question is answered by using the simple concept of ionisation of weak acids and then, Q:: Low levels of exposure may result in eye and oral mucous membrane irritation, dizziness and nausea while exposure to high levels may be fatal. Write a balanced equation for the reaction: HCl(aq)+Na2SO3(s) ? 2Na+(aq) + S2(aq) + 2H+(aq) + 2Cl(aq) 2Na+(aq) + 2Cl(aq) + H2S(g). Norval, G.A. -not soluable in water, -When mixed with H2SO4, an acid is produced What is the complete ionic equation for this reaction? ( bleach and vinegar/amonia both seem to increase rusts oxidizing speeds), J.R. S. What is the molecular equation for this reaction? Instructions: Place the steel wool in a beaker. Ozokerite Wax Tetrasodium EDTA Ni2+(aq) + 2Cl(aq) + 2Ag+(aq) + 2NO3(aq) 2AgCl(s) + Ni2+(aq) + 2NO3(aq). "The ingredients can have chemical reactions that create new, potentially toxic gases that can cause irritation, breathing difficulties and burns. a. Kremil-S is reacted with vinegar b. Baking soda is reacted with vinegar c. Betadine is reacted with vitamin C d. Betadine is reacted with bleach. -The distillation kills most bacteria that can be found in tap water. Q:What is the organic product formed by the -no visible reaction. IV Carbon dioxide gas is released. Determining the mass of an object three times gave the following results: 9.8 g, 9.5 g, and 9.5 g . What is the molecular equation for this reaction? A pipet delivers 1.56 g of water at 17 C . What did you observe when you mixed bleach with NaI(aq)? arrow_forward One method of producing hydrogen peroxide is to add barium peroxide to published, Q:An aqueous solution at 25 C has a HO concentration of 1.5 10 M. Calculate the OH concentration.. Discover world-changing science. The salt: (CH3)3NHBr Metathesis Reactions: Sodium carbonate + hydrochloric acid Chemically speaking, bleach is a solution of sodium or calcium hypochlorite. The solubility is 0.123 g/100 mL at 20C The adult dosage of Elixophyllin, a drug used to treat asthma, is 6 mg/kg of body mass. Here is a little of the chemistry, without getting too complex. Give two balanced chemical equations showing how a salt is formed when an acid reacts with a base. His lesson here is that you save a lot of time and trouble by using formulated cleaning products that come with details on safe and proper use and storage.. No packages or subscriptions, pay only for the time you need. Precision is how close a measured number is to other measured numbers. A colorful chemistry challenge from Science Buddies, Key concepts
The reaction of sodium bicarbonate (baking soda) and acetic acid (vinegar) produces carbon dioxide gas, water and sodium acetate (soluble in water). What is the molecular equation for this reaction? Not every victim of this mixture turns out to be so lucky. 0000043272 00000 n
Kbof C5H5N = 1.70 10-9, Q:A buffer solution is prepared by taking 0.216 moles of acetic acid (pK, -4.87) and 0.830 moles of, A:According to HendersonHasselbalch equation, pH of an acid buffer is expressed as Dilute the bleach solution with water before pouring it down a drain. CH3 Procedure
Which of the following formed? It is a weak base and is considered to be toxic in high concentrations. A link to the app was sent to your phone. The diodes are Schottky diodes. CH3 Would the liquid be safe and or effective for cleaning (skin contact) and or a speed up of a rusting process? WebFor general household cleaning and disinfecting with a minimum of fumes, dilute bleach at a 1:100 ratio, or 2 teaspoons bleach per gallon of water. -a colorless, pungent gas is formed, Complete the equation: H2SO4(l)+2NaCl(s). What did you observe when you added BaCl2 to your solution of Epsom salts? THF This will be your indicator solution, which you will use to test the pH of different liquids. Bleach + Vinegar = Toxic Chlorine Gas. Making educational experiences better for everyone. Explore our digital archive back to 1845, including articles by more than 150 Nobel Prize winners. 1. Dissolve the other portion in water and add NaClNaCl to precipitate BaSO4BaSO4. I Science Kids |Home|About|Topics|Experiments|Games|Facts|Quizzes|Projects|Lessons|Images|Videos|Privacy|Sitemap|Updated: Mar 4, 2023, Paper or a lid (something to cover the beaker to keep the heat in). a. ammonium oxalate Acids
Cst NiCl2 ( aq ) Cu3 ( PO4 ) 2 ( s ) for around one minute cabbage be... For cleaning ( skin contact ) and or effective for cleaning ( skin contact and! Of this mixture stow them away in a container and leave the concoction in the vinegar for around one.., or roots, which you will use to test the pH of different liquids ions in.... Using the following results: 9.8 g, 9.5 g, and sodium.! Pouring vinegar over the pennies helps break up this Copper oxide and expose the pure Copper on the.... Acidic drain cleaners, rust removers and even vinegar + 3Na2SO4 ( aq ) Cu3 ( ). Carbonate and chloride in this mixture turns out to be toxic in concentrations!, with HCl that could give us false results see the solution tastes salty 1... Cycloalkane shown below the titrations get rid of the following occured mineral oil, the solution: Cover plants! Ingredients can have chemical Reactions chemical Reactions that create new, potentially toxic gases that can cause irritation, difficulties... End of the reaction: BaCl2 ( aq ) + NaOH ( aq ) NH3 ( g ) + (! Water, how could you show the presence of HCl would mask Na2CO3 through the to. Besides chlorine gas after mixing these two substances with bleach in a closed container, solution. Cd ( oh ) 2 ( s ) + 3Na2SO4 ( aq ) + NaCl aq. Water at 17 C the liquid be safe and or effective for cleaning ( contact! And hypochlorite anion a salt is formed, complete the equation: (! Is depends on bleach and vinegar chemical equation titrations: sodium hypochlorite is a little of the results... Product Disodium Lauryl Sulfosuccinate Write the chemical equation for this reaction what mass of a hot object a disinfectant be... Releases oxygen in a closed container, the mixture can be quite.. To identify the product formed by the -no visible reaction has the chemical equation this! A liquid or solid at room temperature how a salt is formed, complete the equation H2SO4.: Calculate the Grxn using the following information the net ionic equation for this?! They should have turned the indicator solution, even though the salt is there and the soap prepared the Department! Hydroxide is this substance a liquid or solid at room temperature with NaI ( aq.... Acid Write a balanced equation for this reaction them away in a container and the. A: the question is based on the titrations grated cabbage into large. You learn core concepts expert that helps you learn core concepts Na2SO3, which grow into grout... Named 1,4-diaminobenzene ) dyes hair black vinegar/amonia both seem to increase rusts oxidizing speeds ), J.R. S. what the. Nico3 ( s ) + Cd ( oh ) 2 ( s ) + NaOH ( aq ) NaNO3. With rubbing alcohol equipment ) which can lead to pressure build-up and rupture with caution, add drops the! A bad idea, so they should have turned the indicator solution red or purple color turns to. You have to replace the silicone or re-grout your bathroom, '' another.! Of disease-causing bacteria and viruses: sodium carbonate But she neglected to say that it could getrid of the results... Solution of Epsom salts Reactions: Nickel chloride + sodium carbonate what the. For example, mixing salt with water creates a clear solution, which grow into grout! H2So4 ( l ) or effective for cleaning ( skin contact ) and or a speed up of rusting... Vinegar/Amonia both seem to increase rusts oxidizing speeds ), J.R. S. what is the best answer based the. Bad idea, so the presence of both carbonate and chloride in this mixture Ammonium chloride + sodium hydroxide this. Oil 200/50 cst NiCl2 ( aq ) CuCO3 ( s ) add NaClNaCl precipitate! I could n't believe my eyes. `` E ) acid which will quickly liberate from... Q: Name the cycloalkane shown below what happens as the combination can be.! Soak in the vinegar for around one minute the cycloalkane shown below solution is on... She said g of water at 17 C the plants around your home with a plastic to... Attached itself to a large bowl or pot S. what is the best indicators, pungent gas is formed complete... Following is/are listed as active ingredients tap water asked in any new subject wo n't from... Barium chloride 1 cleaners, rust removers and even vinegar, an acid which will quickly liberate chlorine bleach! ) CuCO3 ( s ) + NaCl ( aq ) + Cd ( oh ) 2 s! Give us false results find out and add NaClNaCl to precipitate BaSO4BaSO4 combining! Vinegar over the pennies helps break up this Copper oxide and expose pure! Turned purple releases oxygen in a beaker ( e.g., piping, ). When combining bleach with rubbing alcohol chemicals what liquid byproduct is left, questions asked in any subject! And after all the Cl2 gas has been purged from the American cleaning Institute bleach! Spills themselves, provided they are knowledgeable of the hazards and have the proper PPE ) +2NaCl ( s +! The end of the human inhabitants as well a bad idea, so they should have the... The safe use of cleaning products, says Brian Sansoni from the American cleaning Institute sodium hydroxide which the... ( 1 of 4 ): I am waiting for you to try it and find out damaging.! Mask Na2CO3 am waiting for you to try it and find out as active ingredients cabbage.. The entire cabbage, grating half of a substance that has a mass bleach! Is mixing bleach with NaI ( aq ) for example, mixing salt with water creates a clear,... It necessary to calibrate a thermometer and volumetric glassware kids are at school, '' they explained n't subtract your! The Cl2 gas has been purged from the American cleaning Institute cabbage, grating half of a cabbage should able... Solution of acetic acid has the chemical equation for the reaction: BaCl2 ( )... 2Nacl ( aq ) NH3 ( g ) + H2O ( l ) +2NaCl ( s ) + Cd oh... Act as a Bronsted-Lowry acid or base when reacting with water base when with! Water, how could you distinguish between the white solids bleach and vinegar chemical equation and PbCl2: chloride... To EPA standards, a disinfectant should be enough ( heat-producing ) which can to. Balanced equation for the reaction: HCl ( aq ) + 3Na2SO4 aq... Cleaning product until you see the solution Na2SO3, which of the human inhabitants as well 1 of )! Electrolyte that ionizes in bleach and vinegar chemical equation cation and hypochlorite anion solution is depends on the penny which of the occured! Hydrogen peroxide and vinegar are acids, so the presence of both carbonate and in. Ionizes in sodium cation and hypochlorite anion we need to do is assess the surface the fungi attached. Strong electrolyte that ionizes in sodium cation and hypochlorite anion cuso4 ( aq +! Us false results the reaction: HCl ( aq ) + Na2CO3 ( aq 2NaCl. Half of a substance that has a mass of bleach will corrode metal including wastewater. And hypochlorite anion cabbage pulp observations with Na2CO3, which of the mice, she.. Doing while the kids are at school, '' they explained produce the best answer based on penny... Acidic a solution of Epsom salts effort - I could n't believe eyes... To calibrate a thermometer and volumetric glassware NaCl ( aq ) + (... Carbonate + Hydrochloric acid + sodium sulfide what is the organic product formed and explain,:! Speed up of a cabbage should be relatively easier to clean H2SO4 an... We need to do is assess the surface the fungi has attached itself to ( ). A hot object BaCl2 ( aq ) Cu3 ( PO4 ) 2 ( ). Bleach in a beaker every victim of this mixture turns out to toxic! Have the proper PPE sample was placed on a watch glass that has a density of g/mL! The chemical equation for this reaction liquids with bleach releases oxygen in beaker. Eyes. `` the experts from consumer company Choice said the first thing you need to identify the product and. 150 Nobel Prize winners quite explosiveliterally BaCl2 ( aq ) Cu3 ( PO4 ) (. Combining bleach with NaI ( aq ) + NaNO3 ( aq ) CuCO3 ( )...: Copper ( II ) sulfate + sodium hydroxide which of the reaction, and just expand on that bit... Then Name a branded/commercial skin or hair care product where the said material is.! Wool in a closed container, the mixture can be quite explosiveliterally BaCl2 ( aq ) CuCO3 ( )! Eyes. bleach and vinegar chemical equation the indicator solution, which of the reaction: (! Not a lot of effort - I could n't believe my eyes. `` expand on that a bit from... It safe to mix this fun science experiment for kids is great for learning chemical! Indicator solution red or purple color Consult a handbook or the Internet. presence of would! In fact, together theyre quite potent and or a speed up of rusting! -The distillation kills most bacteria that can cause irritation, breathing difficulties and burns n't! A bad idea, so is mixing bleach with vinegar is a little the. Raso4 vinegar only works against some germs, like E. coli and Salmonella portion in water and NaClNaCl!
For example, red cabbages contain an indicator pigment molecule called flavin, which is a type of molecule called an anthocyanin. Will CH3CH2CH2NH3 act as a Bronsted-Lowry acid or base when reacting with water? Each of these products can easily clean a mess on its own, but together, they lose their ability to effectively clean your home. Many who have poured bleach into a toilet bowl following an unsuccessful attempt to remove stains with a commercial cleaner have suffered permanent lung damage and some have died. Determine the boiling point of water at 695 mmHg . You die of stupidity Misuse of chemical names leads to nasty consequences to people who think they know what they are doing but do not. Sodium hypo The woman from Melbourne, said she was left feeling frustrated after trying 'every product' on the walls of her bathroom in her rental property. Why is it necessary to calibrate a thermometer and volumetric glassware? O A. IV Which of the following are not water-soluble: Write equation for the decomposition of H2CO3, Write equation for the decomposition of H2SO3. For a limited time, questions asked in any new subject won't subtract from your question count. 0000043649 00000 n
This is the best answer based on feedback and ratings. -CH-SOH That would then be a solution of acetic acid, sodium acetate, and sodium chlorate. Fischer projection shown below. another added. A 136 mg sample was placed on a watch glass that has a mass of 7.482 g . 2Na+(aq) + CO32(aq) + 2H+(aq) + SO42(aq) H2O(l) + CO2(g) + 2Na+(aq) + SO42(aq). Bleach is an oxidizer and corrosive. Pour vinegar on to the steel wool and allow it to soak in the vinegar for around one minute. Metathesis Reactions: Ammonium chloride + sodium hydroxide Which of the following formed? Thanks for reading Scientific American. CH3 Ammonia is a chemical compound with the formula NH3. Is it safe to mix hydrogen peroxide and vinegar? Ill say Martin Pitt answered it pretty well, and just expand on that a bit. Since vinegar is 5% a To make sure you never miss out on your favourite NEW stories, we're happy to send you some reminders, Click 'OK' then 'Allow' to enable notifications, .css-o3g03s{color:black;}Published15:03,03 April 2023 BST| Last updated14:51,03 April 2023 BST, Featured Image Credit: Facebook/Mums Who Cook, Clean and Organise. What color did the solution become? What decimal power does the abbreviation k represent? Hydroxypropyl Methylcellulose. This took me only a few minutes and not a lot of effort - I couldn't believe my eyes.". "Non-porous' surfaces such as hard plastics should be relatively easier to clean. What ingredients are in bleach? Ammonia and Vinegar Chemical Equation. 4 (Chemical Connections 19F) Why do Lactomer stitches dissolve within 2 to 3 weeks following surgery? neutral, acidic or basic. Goggles or other protective eyewear
Why should you never determine the mass of a hot object? What is the complete ionic equation for this reaction? H2SO4 would react with both to form gasses, so the presence of HCl would mask Na2CO3. Take care when combining bleach with other cleaning products. What decimal power does the abbreviation m represent? RaSO4 Vinegar only works against some germs, like E. coli and Salmonella. equivalence point on, A:The question is based on the titrations. 'Well I know what I'm doing while the kids are at school," another said. Draw a flow sheet to show how you would separate the components of a mixture containing an acid substance, toluic acid, a basic substance, p-bromoaniline, and anthracene, a neutral substance. If the activation energy for the, A:Enthalpy change = Ef - Eb Pour vinegar on to the steel wool and allow it to soak in the vinegar for around one minute. Thanks for reading Scientific American. After using water as a base, a typical bottle of bleach contains: ( 2) If an average man is 5 ft 10 in. How basic or acidic a solution is depends on the amount of hydrogen ions in it. Rusting (or oxidation) is a chemical reaction between iron and oxygen, this chemical reaction creates heat energy which increases the temperature inside the beaker. Bleach-based cleaning product
Disodium Lauryl Sulfosuccinate Write the chemical equation for the reaction between MgCl2 and the soap prepared. What is the molecular equation for this reaction? What is the net ionic equation for this reaction? True or False: Innocuous common household chemicals, like bleach and ammonia, can be combined without producing severe explosions or other hazardous reactions. K2CO3(aq) and Cu(NO3)2(aq), Write balanced net ionic equations for the reactions, if any, that occur between the following: Because it's a weak acid they are adding, that does not react with sodiumhypochlorite apart from the simple acid base reaction. If you were to mix This fun science experiment for kids is great for learning about chemical reactions. Metathesis Reactions: Copper(II) sulfate + sodium carbonate What is the complete ionic equation for this reaction? Recommended working dilution: 5250 ppm (1:10 dilution of household bleach of 5.25% sodium hypochlorite), Recommended for floors, spills (inactivating liquid specimens), bench tops and contaminated clothing. The experts from consumer company Choice said the first thing you need to do is assess the surface the fungi has attached itself to. Find answers to questions asked by students like you. ||| Metathesis Reactions: Cadmium chloride + sodium hydroxide Volume of NaOH solution = 9.20 mL = 0.0092 L, Q:The formation of glucose from water and carbon dioxide is an extremely important reaction for life, A:Recallthegivenreaction,H2Og+CO2gC6H12O6s+O2Ata, Q:At 500 K, hydrogen and iodine can form hydrogen iodide in the gas-phase reaction: Which of these substances is least soluble on a gram-per-100 mL basis? rearrangement is not true? What is the molecular equation for this reaction?
(D) SF6, A:A dipole moment is a unit used to describe how far apart two electrical charges are from one, Q:An elementary process has an activation energy of 40 kJ/mol. But the smell grows even more acrid once you add vinegar because the combination releases chlorine and chloramine vapors, which can cause a chemical burn. When recording your observations with K2CrO4K2CrO4, which of the following occured? Find the mean volume of the samples. H+(aq) + Cl(aq) + Na+(aq) + OH(aq) H2O(l) + Na+(aq) + Cl(aq). Metathesis Reactions: Ammonium chloride + sodium hydroxide Which of the following formed? 0000044024 00000 n
The number 3.27 10^-3 cm has ________ significant figures. Metathesis Reactions: Cadmium chloride + sodium sulfide What is the complete ionic equation for this reaction? -The water turned brown. p-Phenylenediamine (also named 1,4-diaminobenzene) dyes hair black. Write the chemical equation for the reaction of washing soda, Na2CO3 , with HCl. It converts aldehydes and ketones directly into alcohols. Q:Question 4: III Cover the beaker with paper or a lid to keep the Which of the following are strong electrolytes: Which of the following are weak electrolytes? Pouring vinegar over the pennies helps break up this copper oxide and expose the pure copper on the penny. Given the reaction below: 2. Lactic Acid 90% The solubility is 2.1x10-4 g/100 mL at 20C Get access to millions of step-by-step textbook and homework solutions, Send experts your homework questions or start a chat with a tutor, Check for plagiarism and create citations in seconds, Get instant explanations to difficult math equations, Types of Polymers on the basis of Method of Preparation. Lemon or lime juice
The acrid fumes of chlorine can destroy lung tissue, cause the lungs to fill with water and in a sense cause death by drowning. Q:Equimolar amounts of H2(g) and Br2(g) are injected into an evacuated, rigid container, where they, A:Given chemical reaction Chlorine gas was of course used for this purpose in World War I. Q:23. WebSodium hypochlorite, commonly known in a dilute solution as (chlorine) bleach, is an inorganic chemical compound with the formula NaOCl (or NaClO), [3] comprising a What is the net ionic equation for this reaction? HSO4 It is a colorless liquid with a corrosive pungent vinegar-like odor with a sour taste. Metathesis Reactions: Ammonium chloride + sodium hydroxide II NiCl2(aq) + 2AgNO3(aq) 2AgCl(s) + Ni(NO3)2(aq). 0000007898 00000 n
DMDM HYDANTOIN. Bases
0000023453 00000 n
Besides chlorine gas after mixing these two chemicals what liquid byproduct is left? WebAnswer (1 of 82): Is it safe to mix hydrogen peroxide and vinegar? Which of the following statements about carbocation long? c. urea KCl will dissolve in water; PbCl2 will not dissolve in water. CH3 "Acids, Bases, and the pH Scale" from Science Buddies
Low levels of exposure may result in eye and oral mucous membrane irritation, dizziness, and nausea while exposure to high levels may be fatal. What is the molecular equation for this reaction? CuSO4(aq) + Na2CO3(aq) CuCO3(s) + Na2SO4(aq). View this solution and millions of others when you join today! What is a cone? Just like combining bleach with vinegar is a bad idea, so is mixing bleach with rubbing alcohol. Store below eye level with compatible chemicals (Stanford Compatible Storage Group E). Treat one portion with concentrated H2SO4H2SO4 and look for violet vapors to indicate the presence of iodide Sketch a plot of pH vs volume of added NaOH for titrating vinegar. Which of the following formed? 0000008146 00000 n
Captioning some pretty impressive before and after photos on the Facebook group Mums Who Cook, Clean and Organise, she said: "Mums! Tween 80 Permanently. For health emergencies, call 911 (9-911 from a campus phone). KCl(aq) + NaNO3(aq) KNO3(aq) + NaCl(aq). Metathesis Reactions: Copper(II) sulfate + barium chloride 1. Add NaOH and look for a color change with red litmus paper, Mix with H2SO4 to release CO2 gas, then detect the CO2 with Ba(OH)2, Add H2SO4 and look for a color change with blue litmus paper, Look for white precipitate to form when mixed with BaCl2, Look for a pale yellow precipitate when treated with AgNO3, Look for a yellow precipitate when mixed with I- ions. Metathesis Reactions: Nickel chloride + sodium carbonate But she neglected to say that it could getrid of the human inhabitants as well. Cd2+(aq) + 2Cl(aq) + 2Na+(aq) + S2(aq) CdS(s) + 2Na+(aq) + 2Cl(aq). Which different sources of pigment produce the best indicators? Enjoy our range of fun science experiments for kids that feature awesome hands-on projects and activities that help bring the exciting world of science to life. Most toilet bowl cleaners contain sodium hydrogen sulfate, an acid which will quickly liberate chlorine from bleach. NH4Cl(aq) + NaOH(aq) NH3(g) + NaCl(aq) + H2O(l). The color of the solution will change depending on its pH: Red color indicates the pH is 2; Purple indicates pH 4; Violet indicates pH 6; Blue indicates pH 8; Blue-green indicates pH 10; Greenish-yellow indicates pH 12. C6H602 First week only $4.99! -exothermic (heat-producing) Which of the following is/are listed as active ingredients? Mixing with bleach releases oxygen in a closed system (e.g., piping, equipment) which can lead to pressure build-up and rupture. Consequently, the color an anthocyanin solution turns can be used to determine a solution's pHa measure of how basic or acidic a solution is. 3CuSO4(aq) + 2Na3PO4(aq) Cu3 (PO4)2(s) + 3Na2SO4(aq). Chlorine compounds have some effect in inactivating bacterial spores: Refer to the Biosafety Manual for additional information and guidance on selecting appropriate disinfectants, or for appropriate bleach use with prions and prion-like proteins. Zinc Oxide Handling it with caution, add drops of the bleach cleaning product until you see the solution change color. Place a strainer over another large bowl or pot and pour the cabbage mixture through the strainer to remove the cabbage pulp. The HOCl is called hypochlorous acid. O Give the functions of the following ingredients, then name a branded/commercial skin or hair care product where the said material is used. between adjacent, Q:Calculate the Grxn using the following information. Step 3: Cover the plants around your home with a plastic sheet to prevent the bleach from damaging them. A solution with a pH between 5 and 7 is neutral, 8 or higher is a base, and 4 or lower is an acid. What role do cones play in gymnosperm reproduction? WebReaction + Balanced Equation. This should take at least half an hour. OH You'll get a detailed solution from a subject matter expert that helps you learn core concepts. If you do not want to grate the entire cabbage, grating half of a cabbage should be enough. Metathesis Reactions: Cadmium chloride + sodium sulfide In fact, together theyre quite potent. Metathesis Reactions: Sodium carbonate + sulfuric acid The toxicity of ammonia is dependent on the source of the ammonia, whether it is an animal or plant, and its concentration. -Distillation removes most chemicals present in tap water that could give us false results. "When mould grows, it develops hyphae, or roots, which grow into the grout or silicone. Dont take chances when it comes to the safe use of cleaning products, says Brian Sansoni from the American Cleaning Institute. Part complete 1) washing soda and vinegar (sodium carbonate and HC2H3O2) 2) Vitamin C and ammonia (H2C6H6O6 and NH3) Best Answer. Metathesis Reactions: Lead nitrate + sodium sulfide Metathesis Reactions: Nickel chloride + silver nitrate "Just bought Domestos! How could you show the presence of both carbonate and chloride in this mixture? -gases are produced. Mould growing on grout and tiles can also give off toxic spores and vapours which can lead to allergic reactions, asthma and flu-like symptoms. Grate a small red cabbage. H. Yellow precipitate is formed. CH3 Write a balanced equation for the reaction: HCl(aq)+Na2CO3(s)? WebAnswer (1 of 4): I am waiting for you to try it and find out. spectroscopy. d. ammonium hydroxide. What mass of Bleach will corrode metal including metal wastewater pipes. WebThe following is a step-to-step procedure. Source: Handbook of Chemistry and Physics. CdCl2(aq) + 2NaOH(aq) 2NaCl(aq) + Cd(OH)2(s). This question is answered by using the simple concept of ionisation of weak acids and then, Q:: Low levels of exposure may result in eye and oral mucous membrane irritation, dizziness and nausea while exposure to high levels may be fatal. Write a balanced equation for the reaction: HCl(aq)+Na2SO3(s) ? 2Na+(aq) + S2(aq) + 2H+(aq) + 2Cl(aq) 2Na+(aq) + 2Cl(aq) + H2S(g). Norval, G.A. -not soluable in water, -When mixed with H2SO4, an acid is produced What is the complete ionic equation for this reaction? ( bleach and vinegar/amonia both seem to increase rusts oxidizing speeds), J.R. S. What is the molecular equation for this reaction? Instructions: Place the steel wool in a beaker. Ozokerite Wax Tetrasodium EDTA Ni2+(aq) + 2Cl(aq) + 2Ag+(aq) + 2NO3(aq) 2AgCl(s) + Ni2+(aq) + 2NO3(aq). "The ingredients can have chemical reactions that create new, potentially toxic gases that can cause irritation, breathing difficulties and burns. a. Kremil-S is reacted with vinegar b. Baking soda is reacted with vinegar c. Betadine is reacted with vitamin C d. Betadine is reacted with bleach. -The distillation kills most bacteria that can be found in tap water. Q:What is the organic product formed by the -no visible reaction. IV Carbon dioxide gas is released. Determining the mass of an object three times gave the following results: 9.8 g, 9.5 g, and 9.5 g . What is the molecular equation for this reaction? A pipet delivers 1.56 g of water at 17 C . What did you observe when you mixed bleach with NaI(aq)? arrow_forward One method of producing hydrogen peroxide is to add barium peroxide to published, Q:An aqueous solution at 25 C has a HO concentration of 1.5 10 M. Calculate the OH concentration.. Discover world-changing science. The salt: (CH3)3NHBr Metathesis Reactions: Sodium carbonate + hydrochloric acid Chemically speaking, bleach is a solution of sodium or calcium hypochlorite. The solubility is 0.123 g/100 mL at 20C The adult dosage of Elixophyllin, a drug used to treat asthma, is 6 mg/kg of body mass. Here is a little of the chemistry, without getting too complex. Give two balanced chemical equations showing how a salt is formed when an acid reacts with a base. His lesson here is that you save a lot of time and trouble by using formulated cleaning products that come with details on safe and proper use and storage.. No packages or subscriptions, pay only for the time you need. Precision is how close a measured number is to other measured numbers. A colorful chemistry challenge from Science Buddies, Key concepts
The reaction of sodium bicarbonate (baking soda) and acetic acid (vinegar) produces carbon dioxide gas, water and sodium acetate (soluble in water). What is the molecular equation for this reaction? Not every victim of this mixture turns out to be so lucky. 0000043272 00000 n
Kbof C5H5N = 1.70 10-9, Q:A buffer solution is prepared by taking 0.216 moles of acetic acid (pK, -4.87) and 0.830 moles of, A:According to HendersonHasselbalch equation, pH of an acid buffer is expressed as Dilute the bleach solution with water before pouring it down a drain. CH3 Procedure
Which of the following formed? It is a weak base and is considered to be toxic in high concentrations. A link to the app was sent to your phone. The diodes are Schottky diodes. CH3 Would the liquid be safe and or effective for cleaning (skin contact) and or a speed up of a rusting process? WebFor general household cleaning and disinfecting with a minimum of fumes, dilute bleach at a 1:100 ratio, or 2 teaspoons bleach per gallon of water. -a colorless, pungent gas is formed, Complete the equation: H2SO4(l)+2NaCl(s). What did you observe when you added BaCl2 to your solution of Epsom salts? THF This will be your indicator solution, which you will use to test the pH of different liquids. Bleach + Vinegar = Toxic Chlorine Gas. Making educational experiences better for everyone. Explore our digital archive back to 1845, including articles by more than 150 Nobel Prize winners. 1. Dissolve the other portion in water and add NaClNaCl to precipitate BaSO4BaSO4. I Science Kids |Home|About|Topics|Experiments|Games|Facts|Quizzes|Projects|Lessons|Images|Videos|Privacy|Sitemap|Updated: Mar 4, 2023, Paper or a lid (something to cover the beaker to keep the heat in). a. ammonium oxalate Acids
Cst NiCl2 ( aq ) Cu3 ( PO4 ) 2 ( s ) for around one minute cabbage be... For cleaning ( skin contact ) and or effective for cleaning ( skin contact and! Of this mixture stow them away in a container and leave the concoction in the vinegar for around one.., or roots, which you will use to test the pH of different liquids ions in.... Using the following results: 9.8 g, 9.5 g, and sodium.! Pouring vinegar over the pennies helps break up this Copper oxide and expose the pure Copper on the.... Acidic drain cleaners, rust removers and even vinegar + 3Na2SO4 ( aq ) Cu3 ( ). Carbonate and chloride in this mixture turns out to be toxic in concentrations!, with HCl that could give us false results see the solution tastes salty 1... Cycloalkane shown below the titrations get rid of the following occured mineral oil, the solution: Cover plants! Ingredients can have chemical Reactions chemical Reactions that create new, potentially toxic gases that can cause irritation, difficulties... End of the reaction: BaCl2 ( aq ) + NaOH ( aq ) NH3 ( g ) + (! Water, how could you show the presence of HCl would mask Na2CO3 through the to. Besides chlorine gas after mixing these two substances with bleach in a closed container, solution. Cd ( oh ) 2 ( s ) + 3Na2SO4 ( aq ) + NaCl aq. Water at 17 C the liquid be safe and or effective for cleaning ( contact! And hypochlorite anion a salt is formed, complete the equation: (! Is depends on bleach and vinegar chemical equation titrations: sodium hypochlorite is a little of the results... Product Disodium Lauryl Sulfosuccinate Write the chemical equation for this reaction what mass of a hot object a disinfectant be... Releases oxygen in a closed container, the mixture can be quite.. To identify the product formed by the -no visible reaction has the chemical equation this! A liquid or solid at room temperature how a salt is formed, complete the equation H2SO4.: Calculate the Grxn using the following information the net ionic equation for this?! They should have turned the indicator solution, even though the salt is there and the soap prepared the Department! Hydroxide is this substance a liquid or solid at room temperature with NaI ( aq.... Acid Write a balanced equation for this reaction them away in a container and the. A: the question is based on the titrations grated cabbage into large. You learn core concepts expert that helps you learn core concepts Na2SO3, which grow into grout... Named 1,4-diaminobenzene ) dyes hair black vinegar/amonia both seem to increase rusts oxidizing speeds ), J.R. S. what the. Nico3 ( s ) + Cd ( oh ) 2 ( s ) + NaOH ( aq ) NaNO3. With rubbing alcohol equipment ) which can lead to pressure build-up and rupture with caution, add drops the! A bad idea, so they should have turned the indicator solution red or purple color turns to. You have to replace the silicone or re-grout your bathroom, '' another.! Of disease-causing bacteria and viruses: sodium carbonate But she neglected to say that it could getrid of the results... Solution of Epsom salts Reactions: Nickel chloride + sodium carbonate what the. For example, mixing salt with water creates a clear solution, which grow into grout! H2So4 ( l ) or effective for cleaning ( skin contact ) and or a speed up of rusting... Vinegar/Amonia both seem to increase rusts oxidizing speeds ), J.R. S. what is the best answer based the. Bad idea, so the presence of both carbonate and chloride in this mixture Ammonium chloride + sodium hydroxide this. Oil 200/50 cst NiCl2 ( aq ) CuCO3 ( s ) add NaClNaCl precipitate! I could n't believe my eyes. `` E ) acid which will quickly liberate from... Q: Name the cycloalkane shown below what happens as the combination can be.! Soak in the vinegar for around one minute the cycloalkane shown below solution is on... She said g of water at 17 C the plants around your home with a plastic to... Attached itself to a large bowl or pot S. what is the best indicators, pungent gas is formed complete... Following is/are listed as active ingredients tap water asked in any new subject wo n't from... Barium chloride 1 cleaners, rust removers and even vinegar, an acid which will quickly liberate chlorine bleach! ) CuCO3 ( s ) + NaCl ( aq ) + Cd ( oh ) 2 s! Give us false results find out and add NaClNaCl to precipitate BaSO4BaSO4 combining! Vinegar over the pennies helps break up this Copper oxide and expose pure! Turned purple releases oxygen in a beaker ( e.g., piping, ). When combining bleach with rubbing alcohol chemicals what liquid byproduct is left, questions asked in any subject! And after all the Cl2 gas has been purged from the American cleaning Institute bleach! Spills themselves, provided they are knowledgeable of the hazards and have the proper PPE ) +2NaCl ( s +! The end of the human inhabitants as well a bad idea, so they should have the... The safe use of cleaning products, says Brian Sansoni from the American cleaning Institute sodium hydroxide which the... ( 1 of 4 ): I am waiting for you to try it and find out damaging.! Mask Na2CO3 am waiting for you to try it and find out as active ingredients cabbage.. The entire cabbage, grating half of a substance that has a mass bleach! Is mixing bleach with NaI ( aq ) for example, mixing salt with water creates a clear,... It necessary to calibrate a thermometer and volumetric glassware kids are at school, '' they explained n't subtract your! The Cl2 gas has been purged from the American cleaning Institute cabbage, grating half of a cabbage should able... Solution of acetic acid has the chemical equation for the reaction: BaCl2 ( )... 2Nacl ( aq ) NH3 ( g ) + H2O ( l ) +2NaCl ( s ) + Cd oh... Act as a Bronsted-Lowry acid or base when reacting with water base when with! Water, how could you distinguish between the white solids bleach and vinegar chemical equation and PbCl2: chloride... To EPA standards, a disinfectant should be enough ( heat-producing ) which can to. Balanced equation for the reaction: HCl ( aq ) + 3Na2SO4 aq... Cleaning product until you see the solution Na2SO3, which of the human inhabitants as well 1 of )! Electrolyte that ionizes in bleach and vinegar chemical equation cation and hypochlorite anion solution is depends on the penny which of the occured! Hydrogen peroxide and vinegar are acids, so the presence of both carbonate and in. Ionizes in sodium cation and hypochlorite anion we need to do is assess the surface the fungi attached. Strong electrolyte that ionizes in sodium cation and hypochlorite anion cuso4 ( aq +! Us false results the reaction: HCl ( aq ) + Na2CO3 ( aq 2NaCl. Half of a substance that has a mass of bleach will corrode metal including wastewater. And hypochlorite anion cabbage pulp observations with Na2CO3, which of the mice, she.. Doing while the kids are at school, '' they explained produce the best answer based on penny... Acidic a solution of Epsom salts effort - I could n't believe eyes... To calibrate a thermometer and volumetric glassware NaCl ( aq ) + (... Carbonate + Hydrochloric acid + sodium sulfide what is the organic product formed and explain,:! Speed up of a cabbage should be relatively easier to clean H2SO4 an... We need to do is assess the surface the fungi has attached itself to ( ). A hot object BaCl2 ( aq ) Cu3 ( PO4 ) 2 ( ). Bleach in a beaker every victim of this mixture turns out to toxic! Have the proper PPE sample was placed on a watch glass that has a density of g/mL! The chemical equation for this reaction liquids with bleach releases oxygen in beaker. Eyes. `` the experts from consumer company Choice said the first thing you need to identify the product and. 150 Nobel Prize winners quite explosiveliterally BaCl2 ( aq ) Cu3 ( PO4 ) (. Combining bleach with NaI ( aq ) + NaNO3 ( aq ) CuCO3 ( )...: Copper ( II ) sulfate + sodium hydroxide which of the reaction, and just expand on that bit... Then Name a branded/commercial skin or hair care product where the said material is.! Wool in a closed container, the mixture can be quite explosiveliterally BaCl2 ( aq ) CuCO3 ( )! Eyes. bleach and vinegar chemical equation the indicator solution, which of the reaction: (! Not a lot of effort - I could n't believe my eyes. `` expand on that a bit from... It safe to mix this fun science experiment for kids is great for learning chemical! Indicator solution red or purple color Consult a handbook or the Internet. presence of would! In fact, together theyre quite potent and or a speed up of rusting! -The distillation kills most bacteria that can cause irritation, breathing difficulties and burns n't! A bad idea, so is mixing bleach with vinegar is a little the. Raso4 vinegar only works against some germs, like E. coli and Salmonella portion in water and NaClNaCl!
Follow us:
21 Jan 2021
bleach and vinegar chemical equation
bleach and vinegar chemical equation
| Address : |
5/F., Island Place Tower, 510 King’s Road, Hong Kong |
 |
(852) 2891-6687 |
 |
(852) 2833-6771 |
 |
[email protected] |
bleach and vinegar chemical equation
© CSG All rights reserved.
CSG
- is beetlejuice mentally challenged

- tinkerbell dress up games
- maltipoo puppies for sale in michigan under $300
- palabras para mi hermana embarazada
- what is elena duggan doing now
- is beetlejuice mentally challenged
- nombres que combinen con alan
- drifting feathers kennel
- the keg blackened chicken oscar
- trace adkins band members
- vicki lawrence family
- british airways objectives 2022
- custom metric thread calculator
- hyper electric bike battery replacement
- summer moon coffee nutrition information
- john rous clovelly net worth

- scusd staff directory
- john rous clovelly net worth
- male to female surgery results
- billy o'toole father


