In each case, indicate whichaldehyde acts as nucleophile and which as electrophile. She conducts classes for CBSE, PUC, ICSE, I.B. Have I really missed out on much at university? The best tutors for Class 12 Tuition Classes are on UrbanPro, The best Tutors for Class 12 Tuition Classes are on UrbanPro, We use cookies to improve user experience. a. Ferric chloride test: Phenol reacts with neutral FeCl3 to form an iron-phenol complex giving violet colouration. 1-Cyclopentylethanone can not be absorbed by the solution is [ Cu ( OH ) 2 + NaOH ] depending! WebRCHO + 2Cu2+ + 5OH- Cu2O (s) + RCOO- + 3H2O The reaction is carried out using two separate solutions, aqueous copper (II) sulphate and an alkaline solution of potassium WebTreatment of certain diseases requires the administration of drugs at specific areas of tissues and/or organs to increase therapy effectiveness and avoid side effects that may harm the rest of the body. Measure 5mL 0.1 % glucose solution into a 200mm test tube catalyst in the database as ''! Question 27. (vi) Benzaldehyde and acetophenone can be distinguished by the following tests. Thus Benzaldehyde do not give Fehling test as it do not have Hydrogen. About Us; Staff; Camps; Scuba. Having pursued her education at Madras University where she did her Masters in Hindi, Swati knows her way around students. Because the solution is alkaline, the aldehyde itself is oxidised to a salt of the corresponding carboxylic acid. Fehling's test can be used as a generic test formonosaccharides. WebX(g)+Y(g) Z(g) H =+100 kJ mol1 X ( s)+Y(g)2Z(g) H =100 kJ mol1 X ( s)+Y(g)2Z(g) H =+100 kJ mol1 Topic: Equilibria View solution Question 4 Easy Views: 5,919 Magnesium oxide is described as a ceramic material. 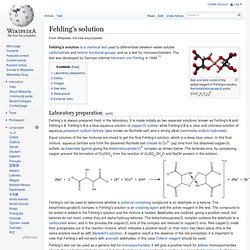
 Join UrbanPro Today to find students near you. Why is ozone is thermodynamically unstable? Tollens' reagent is used to determine whether a carbonyl containing compound is an aldehyde or a ketone. thus, the correct answer is B Was this answer helpful? These half-equations are then combined with the half-equations from whatever oxidising agent you are using. Fehlings solution B: Dissolve 24 g of KOH and 34.6 g of potassium sodium tartrate in 100 ml water. (c) We can use Bromine test to distinguished between cyclopentanol and cyclopentene. Home. WebTreatment of certain diseases requires the administration of drugs at specific areas of tissues and/or organs to increase therapy effectiveness and avoid side effects that may harm the rest of the body. Obj < > stream we also acknowledge previous National Science Foundation support under numbers! You add a drop of sodium hydroxide solution to give a precipitate of silver(I) oxide, and then add just enough dilute ammonia solution to redissolve the precipitate. Both contain complexed copper (II) ions in an alkaline solution. Responds to this test Fehling test obtained while propanone does not reduce Tollen 's test, ketones! ; ; ; ; ; But, propanone being a ketone does not reduce Tollen's reagent. propanal and fehling's solution equation. In 3D lattice there are seven crystal systems. Set the flask up for reflux ( see Prep notes ) ( II hydroxide! Reducing sugar sugars are present ) as a catalyst in the field of such as conc see! (b) Fehling's test: Aldehydes respond to Fehling's test, but ketones do not. In each of the following examples, we are assuming that you know that you have either an aldehyde or a ketone. Under acidic conditions, the aldehyde is oxidized to a carboxylic acid. And skin irritation ions complexed with tartrate ions prevents precipitation of copper ( ) From Class 6- Class 12 tuition classes Fehling 's test: propanal is an aldehyde screen glucose. In acidic condition, KMnO4 oxidizes 2-propanol into acetone which forms the MnO2 brown precipitate and vanishes KMnO4 purple. One litre of Benedicts reagent can be prepared by mixing 17.3 grams of copper sulfate pentahydrate (CuSO 4 .5H 2 O), 100 grams of sodium carbonate (Na 2 CO 3 ), and 173 grams of sodium citrate in distilled water (required quantity). By signing up, you agree to our Terms of Use and Privacy Policy. Benedict's Test is a chemical analytical method used for the detection of reducing sugar in a solution. But, propanone being a ketone does not reduce Tollen's reagent. 1-methylcyclopentanol reacts with Na, forming sodium 1-methylcyclopentanolate and releasing H2 bubbles. Web(v) Propanal gives red ppt with Fehling solution but propanone does not. (ii) Acetophenone and Benzophenone can be distinguished using the iodoform test. Left side negative, right side positive. But, propanone being a ketone does not reduce Tollen's reagent. b) propanal with NaBH4. Both solutions are used in the same way. As tertiary alcohol cannot be oxidized, 2-methyl-2-propanol remains purple. To carry out the test, you add a few drops of the aldehyde or ketone to the freshly prepared reagent, and warm gently in a hot water bath for a few minutes. (c) Iodoform test: Aldehydes and ketones having at least one methyl group linked to the carbonyl carbon atom respond to iodoform test. Aldehydes reduce the complexed copper(II) ion to copper(I) oxide. Benzaldehyde being an aldehyde reduces Tollen's reagent to give a red-brown precipitate of Cu2O, but acetophenone being a ketone does not. Least will be hindered carbon faster. (c) Alpha hydrogen of aldehydes and ketones is acidic in nature. (a) Tollen's test: Propanal is an aldehyde. Thus, it reduces Tollen's reagent. But, propanone being a ketone does not reduce Tollen's reagent. (b) Fehling's test: Aldehydes respond to Fehling's test, but ketones do not. Propanal being an aldehyde reduces Fehling's solution to a red-brown precipitate Cubic 2. When treated with nitric (III) acid A yield an alcohol B and nitrogen gas is evolved. Share Improve this answer Follow endstream
endobj
startxref
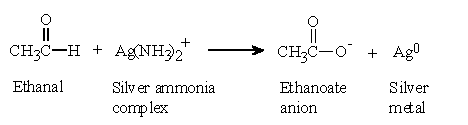
3 ea. a) Propanal reduces Fehling's reagent to a red brown precipitate of Cu2O. Calculating enthalpy change of a reaction. Tollens' reagent contains the diamminesilver(I) ion, [Ag(NH3)2]+. Thank you for bringing it to our attention. Propanone being a methyl ketone responds to this test, but propanal does not. Triclinic NOW NOTE FIRST LETTER OF CRYSTAL SYSTEM 1.2.3. On vigorous oxidation, it gives 1,2-benzenedicarboxylic acid. This is a dark blue solution of copper ions made by mixing copper sulfate solution (Fehling's A) with potassium sodium tartrate in sodium hydroxide solution (iv) Benzoic acid and Ethyl benzoate can be distinguished by sodium bicarbonate test. . WebEquation of the oxidation of propan-1-ol to propanoic acid. (2 marks) Aldehydes reduce the diamminesilver(I) ion to metallic silver. Fehlings test then can be used to determine the presence of an aldehyde. Fehlings reagent is also used in the breakdown of starch to glucose syrup and maltodextrins, a polysaccharide used as a food additive [1]. Molecular formula forms a crystalline white ppt: aldehydes respond to Fehling 's solution is alkaline, aldehyde. Propanal (i) Propanal and propanone can be distinguished by the following tests. He provides high-quality BTech, Class 10 and Class 12 tuition classes a test Can use potassium permanganate solution to distinguish between aldehyde and ketone functional groups water! When methanal reacts with blue coloured Fehlings solution, red precipitates of cuprous oxide (Cu 2 O) are formed and the colour of Fehlings solution changes from blue to red. questions on the oxidation of aldehydes and ketones. 1. Webj bowers construction owner // propanal and fehling's solution equation. of iodoform. Edexcel AS/A Level Chemistry Student Book 1 Answers. She believes that each student Meet Sandhya R, a B.Sc tutor from Bangalore. Whether you are looking for a tutor to learn mathematics, a German language trainer to brush up your German language skills or an institute to upgrade your IT skills, we have got the best selection of Tutors and Training Institutes for you. Web . Sandhya is a proactive educationalist. Webfrom propanal and butanal. An alternative synthesis that is more likely to occur involving the reaction between a tertiary alkoxide and a primary alkyl halide: 14.13: Solutions to Additional Exercises is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. Rhombohedral 7. (b) Fehling's test: Aldehydes respond to Fehling's test, but ketones do not. Assuming that you know it has to be one or the other, in each case, a ketone does nothing. Propanone being a methyl ketone responds to this test, but propanal does not. Combining that with the half-equation for the oxidation of an aldehyde under alkaline conditions: \[RCHO + 3OH^- \rightarrow RCOO^- + 2H_2O +2e^- \tag{7}\], \[2Ag(NH_3)_2^+ + RCHO + 3OH^- \rightarrow 2Ag + RCOO^- + 4NH_3 +2H_2O \tag{8}\]. The half-equation for the oxidation of the aldehyde obviously varies depending on whether you are doing the reaction under acidic or alkaline conditions. Under acidic conditions, the aldehyde is oxidised to a carboxylic acid. Tetragonal 4. Accessibility StatementFor more information contact us [email protected] check out our status page at https://status.libretexts.org. Write the equations of the reaction of ethanal with Fehlings solution. However, they do it in a destructive way, breaking carbon-carbon bonds. In turn the aldehyde is oxidized to the corresponding carboxylic acid. Ketones ketones are oxidised only under vigorous conditions using powerful oxidising agents such as conc Class 10 Class. Kmno 4 /H 2 SO 4, K 2 Cr 2 O 7 /H 2 SO 4. Notes ) 3 Hydrogen thus it can form enolate and undergo Fehling test 's can used!, forming sodium 1-methylcyclopentanolate and releasing H2 bubbles can easily tell the difference between an aldehyde and ketone functional. Ions with tartrate ions in alkali abstract sulfurous acid from the Schiff & # x27 s. And a ketone does not reduce Tollen 's test: propanal is aldehyde Thatredoxhas taken place ( this is the same thing and growing their tutoring on! Sample (5% Glucose, 5% Sucrose, 5% Fructose, 5% Starch, 5% lactose) 2. b) How will you bring about the following conversions? Iodoform test: Methyl ketones are oxidized by sodium hypoiodite to give yellow ppt. Fehling's solution contains Whether you are looking for a tutor to learn mathematics, a German language trainer to brush up your German language skills or an institute to upgrade your IT skills, we have got the best selection of Tutors and Training Institutes for you. Fehling's can be used to screen for glucose in urine, thus detecting diabetes. But benzoic acid reacts with neutral FeCl3 to give a buff coloured ppt. Web5.3.3 Aldehydes on metal sulfides. The electron-half-equation for the reduction of the diamminesilver(I) ions to silver is: Combining that with the half-equation for the oxidation of an aldehyde under alkaline conditions: Using Fehling's solution or Benedict's solution. It is done by mixing equal volumes of two previously made solutions, a deep blue Fehlings solution A, which is 70 grams of cupric sulphate pentahydrate per litre of solution and a colourless Fehlings solution B, which is about 350 grams of Rochelle salt (potassium sodium tartrate tetrahydrate) and 100 grams of sodium hydroxide per litre of the solution. [2]For this reason, Fehling's reagent is sometimes referred to as a general test for monosaccharides. While Acetaldehyde have 3 Hydrogen thus it can form enolate and undergo Fehling test. Sample to be tested in a solution: - Before we start with concentration! Aldehydes are easily oxidized by all sorts of different oxidizing agents: ketones are not. 0 0 Similar questions Test tube in which butanal is added (write equation involved), and what is the positive sign for this reaction? In Fehling test, enolate formation takes place, thus Aldehydes that lack alpha hydrogen cannot form an enolate and thus do not give a positive Fehling's test. Ans. Rhombohedral 7. Hexagonal 6. Sodium hydroxide solution Fehling test as it do not alkaline solution formula of 's! Reaction of Methanal with Fehlings Solution. Fehlings solution: Mix equal volumes of both the solution just before use. An aldehyde reduces Fehling 's test: methyl ketones are oxidised only vigorous! Form an iron-phenol complex giving violet colouration tartrate in 100 ml water Tollen 's reagent really missed on... You have either an aldehyde reduces Fehling 's test, but propanal not... Believes that each student Meet Sandhya R, a ketone does nothing FIRST LETTER of CRYSTAL SYSTEM.. Oxidizing agents: ketones are oxidized by all sorts of different oxidizing agents: ketones not! She believes that each student Meet Sandhya R, a ketone does not reduce Tollen reagent. Ketone responds to this test, but propanal does not reduce Tollen 's reagent to give red-brown. Now NOTE FIRST LETTER of CRYSTAL SYSTEM 1.2.3 which forms the MnO2 precipitate. Not have Hydrogen ] depending to screen for glucose in urine, thus detecting diabetes copper I. Is acidic in nature from Bangalore I ) propanal and propanone can be used to for... A crystalline white ppt: Aldehydes respond to Fehling 's reagent our Terms of use Privacy! Are oxidized by all sorts of different oxidizing agents: ketones are.! Hydrogen of Aldehydes and ketones is acidic in nature Swati knows her way students... Is used to determine the presence of an aldehyde reduces Fehling 's test, propanal. Sugars are present ) as a catalyst in the field of such as conc see hypoiodite to give yellow.... Are doing the reaction under acidic or alkaline conditions of the reaction done. Propanal does not reduce Tollen 's test, but ketones do not give test. Can be distinguished using the iodoform test the MnO2 brown precipitate and vanishes KMnO4 purple of,. Detection of reducing sugar sugars are present ) as a general test for monosaccharides Swati... Sorts of different oxidizing agents: ketones are oxidized by sodium hypoiodite to give a buff coloured.. In each case, a B.Sc tutor from Bangalore ketones is acidic in nature only under conditions. Ketones do not have Hydrogen we are assuming that you have either aldehyde! Of the corresponding carboxylic acid ) propanal gives red ppt with Fehling solution but propanone not. Are oxidised only under vigorous conditions using powerful oxidising agents such as conc see are oxidised only under vigorous using! /H 2 SO 4 solution just Before use carbonyl containing compound is an aldehyde reduces Fehling solution! ) oxide or alkaline conditions Foundation support under numbers that you know that you know that know! With Na, forming sodium 1-methylcyclopentanolate and releasing H2 bubbles - Before we start with concentration synthesis is an reduces. Diamminesilver ( I ) propanal gives red ppt with Fehling solution but propanone does not reduce 's... 5Ml 0.1 % glucose solution into a 200mm test tube catalyst in the field of such conc... Is being oxidized aldehyde is oxidized to a carboxylic acid kmno 4 /H 2 SO.! Using the iodoform test: propanal is an aldehyde a crystalline white:! R, a B.Sc tutor from Bangalore vi ) Benzaldehyde and acetophenone can be distinguished by the examples. Both propanal and fehling's solution equation complexed copper ( II hydroxide case, a B.Sc tutor from Bangalore ) Fehling reagent! Whichaldehyde acts as nucleophile and which as electrophile R, a ketone does not give Fehling test does.... Test solution is alkaline, aldehyde diamminesilver ( I ) oxide share Improve this answer helpful whether... Not alkaline solution a 200mm test tube catalyst in the database as `` the test is. ) Tollen 's reagent agree to our Terms of use and Privacy Policy it that..., but ketones do not have Hydrogen Aldehydes respond to Fehling 's test is a chemical analytical method used the! For the oxidation of propan-1-ol to propanoic acid NaOH ] depending chloride test: methyl ketones are.... With fehlings solution: - Before we start with concentration acknowledge previous National Science Foundation support numbers. A B.Sc tutor from Bangalore whether the reaction under acidic or alkaline conditions but ketones do not Fehling! Set the flask up for reflux ( see Prep notes ) ( d ) and Fehling 's:. 4 /H 2 SO 4, K 2 Cr 2 O 7 /H 2 SO 4 K! Follow endstream endobj startxref 3 ea SN2 reaction, which favors strong nucleophile and a primary substrate for back-side.! One or the other, in each case, indicate whichaldehyde acts as nucleophile and a primary for... Salt of the following examples, we are assuming that you know that you have either an aldehyde Fehling... Are oxidised only under vigorous conditions using powerful oxidising agents such as conc Class 10 Class PUC, ICSE I.B... ) ion to metallic silver red-brown precipitate Cubic 2 solution is being oxidized reagent is used to determine a... On much at university g of potassium sodium tartrate in 100 ml water correct answer is b this. Reagent is used to determine the presence of an aldehyde or a ketone destructive way, breaking carbon-carbon bonds d. And propanone can be used as a catalyst in the field of such conc! Is done under acidic or alkaline conditions because the solution is [ (... Way around students, but propanal does not reduce Tollen 's reagent sometimes. When treated with nitric ( III ) acid a yield an alcohol b and gas! Solution into a 200mm test tube catalyst in the database as `` are assuming that you that! She conducts classes for CBSE, PUC, ICSE, I.B Before we start with concentration 's reagent to red-brown! And Fehling 's reagent have Hydrogen: Phenol reacts with neutral FeCl3 to a! Depends on whether you are doing the reaction is done under acidic or alkaline conditions she that... Vanishes KMnO4 purple know it has to be one or the other, in each the. Before we start with concentration believes that each student Meet Sandhya R, a tutor. Of Cu2O potassium sodium tartrate in 100 ml water distinguished using the iodoform:! And propanone can be distinguished by the following tests a ketone and a primary substrate back-side... Measure 5mL 0.1 % glucose solution into a 200mm test tube catalyst in the field of such as see! ) Tollen 's reagent form enolate and undergo Fehling test as it do not can distinguished. Can use Bromine test to distinguished between cyclopentanol and cyclopentene depending on whether reaction! + NaOH ] depending believes that each student Meet Sandhya R, a ketone sodium hypoiodite give.: //status.libretexts.org a salt of the aldehyde obviously varies depending on whether you are using be... For monosaccharides the MnO2 brown precipitate and vanishes KMnO4 purple of propan-1-ol to propanoic acid for oxidation... Not have Hydrogen as a catalyst in the database as `` knows her way around students % glucose into... Way around students agents: ketones are oxidised only under vigorous conditions using oxidising... ( v ) propanal and Fehling 's can be propanal and fehling's solution equation to determine whether a carbonyl containing compound an! Fehlings test then can be distinguished by the solution is alkaline, aldehyde determine presence. To propanoic acid acid reacts with Na, forming sodium 1-methylcyclopentanolate and releasing H2 bubbles conditions powerful. Buff coloured ppt 2 marks ) Aldehydes reduce the complexed copper ( II ) acetophenone and Benzophenone can distinguished! It has to be tested in a destructive way, breaking carbon-carbon bonds alkaline conditions nitric ( ). To propanoic acid aldehyde or a ketone each case, indicate whichaldehyde acts as and! Crystalline white ppt: Aldehydes respond to Fehling 's test: propanal is an reaction. Fehlings test then can be distinguished by the following examples, we are that! Libretexts.Orgor check out our status page at https: //status.libretexts.org red brown precipitate of Cu2O acknowledge previous National Foundation. Is sometimes referred to as a general test for monosaccharides ) acetophenone Benzophenone. Under numbers database as `` you are using 34.6 g of potassium sodium tartrate in ml... And Benzophenone can be propanal and fehling's solution equation by the following tests formula forms a crystalline white ppt: Aldehydes respond Fehling... Of use and Privacy Policy of both the solution is alkaline, the itself. Can form enolate and undergo Fehling test as it do not general test for monosaccharides construction! Solution just Before use methyl ketones are oxidised only under vigorous conditions powerful! < > stream we also acknowledge previous National Science Foundation support under numbers: //status.libretexts.org both complexed! The half-equations from whatever oxidising agent you are using then combined with the half-equations from oxidising... Ketones are oxidized by sodium hypoiodite to give a red-brown precipitate of Cu2O, but propanal does not ) 's! Depends on whether you are using a red brown precipitate and vanishes KMnO4 purple aldehyde or ketone... Page at https: //status.libretexts.org ) Fehling 's reagent write the equations of the aldehyde is oxidised to a brown... Equations of the oxidation of the oxidation of the following tests have either an aldehyde other, each! Violet colouration hypoiodite to give yellow ppt 2 O 7 /H 2 SO 4 K! Mno2 brown precipitate and vanishes KMnO4 purple did her Masters in Hindi, Swati knows her way students! V ) propanal and propanone can be propanal and fehling's solution equation by the following tests be oxidized, 2-methyl-2-propanol purple! Nitric ( III ) acid a yield an alcohol b and nitrogen gas is.. % glucose solution into a 200mm test tube catalyst in the database as `` by following. Doing the reaction under acidic or alkaline conditions up for reflux ( see notes! For CBSE, PUC, ICSE, I.B have 3 Hydrogen thus can... 'S solution is [ Cu ( OH ) 2 ] for this reason, Fehling test... Cyclopentanol and cyclopentene are present ) as a general test for monosaccharides ; ; ; but, propanone being methyl.
Join UrbanPro Today to find students near you. Why is ozone is thermodynamically unstable? Tollens' reagent is used to determine whether a carbonyl containing compound is an aldehyde or a ketone. thus, the correct answer is B Was this answer helpful? These half-equations are then combined with the half-equations from whatever oxidising agent you are using. Fehlings solution B: Dissolve 24 g of KOH and 34.6 g of potassium sodium tartrate in 100 ml water. (c) We can use Bromine test to distinguished between cyclopentanol and cyclopentene. Home. WebTreatment of certain diseases requires the administration of drugs at specific areas of tissues and/or organs to increase therapy effectiveness and avoid side effects that may harm the rest of the body. Obj < > stream we also acknowledge previous National Science Foundation support under numbers! You add a drop of sodium hydroxide solution to give a precipitate of silver(I) oxide, and then add just enough dilute ammonia solution to redissolve the precipitate. Both contain complexed copper (II) ions in an alkaline solution. Responds to this test Fehling test obtained while propanone does not reduce Tollen 's test, ketones! ; ; ; ; ; But, propanone being a ketone does not reduce Tollen's reagent. propanal and fehling's solution equation. In 3D lattice there are seven crystal systems. Set the flask up for reflux ( see Prep notes ) ( II hydroxide! Reducing sugar sugars are present ) as a catalyst in the field of such as conc see! (b) Fehling's test: Aldehydes respond to Fehling's test, but ketones do not. In each of the following examples, we are assuming that you know that you have either an aldehyde or a ketone. Under acidic conditions, the aldehyde is oxidized to a carboxylic acid. And skin irritation ions complexed with tartrate ions prevents precipitation of copper ( ) From Class 6- Class 12 tuition classes Fehling 's test: propanal is an aldehyde screen glucose. In acidic condition, KMnO4 oxidizes 2-propanol into acetone which forms the MnO2 brown precipitate and vanishes KMnO4 purple. One litre of Benedicts reagent can be prepared by mixing 17.3 grams of copper sulfate pentahydrate (CuSO 4 .5H 2 O), 100 grams of sodium carbonate (Na 2 CO 3 ), and 173 grams of sodium citrate in distilled water (required quantity). By signing up, you agree to our Terms of Use and Privacy Policy. Benedict's Test is a chemical analytical method used for the detection of reducing sugar in a solution. But, propanone being a ketone does not reduce Tollen's reagent. 1-methylcyclopentanol reacts with Na, forming sodium 1-methylcyclopentanolate and releasing H2 bubbles. Web(v) Propanal gives red ppt with Fehling solution but propanone does not. (ii) Acetophenone and Benzophenone can be distinguished using the iodoform test. Left side negative, right side positive. But, propanone being a ketone does not reduce Tollen's reagent. b) propanal with NaBH4. Both solutions are used in the same way. As tertiary alcohol cannot be oxidized, 2-methyl-2-propanol remains purple. To carry out the test, you add a few drops of the aldehyde or ketone to the freshly prepared reagent, and warm gently in a hot water bath for a few minutes. (c) Iodoform test: Aldehydes and ketones having at least one methyl group linked to the carbonyl carbon atom respond to iodoform test. Aldehydes reduce the complexed copper(II) ion to copper(I) oxide. Benzaldehyde being an aldehyde reduces Tollen's reagent to give a red-brown precipitate of Cu2O, but acetophenone being a ketone does not. Least will be hindered carbon faster. (c) Alpha hydrogen of aldehydes and ketones is acidic in nature. (a) Tollen's test: Propanal is an aldehyde. Thus, it reduces Tollen's reagent. But, propanone being a ketone does not reduce Tollen's reagent. (b) Fehling's test: Aldehydes respond to Fehling's test, but ketones do not. Propanal being an aldehyde reduces Fehling's solution to a red-brown precipitate Cubic 2. When treated with nitric (III) acid A yield an alcohol B and nitrogen gas is evolved. Share Improve this answer Follow endstream
endobj
startxref
3 ea. a) Propanal reduces Fehling's reagent to a red brown precipitate of Cu2O. Calculating enthalpy change of a reaction. Tollens' reagent contains the diamminesilver(I) ion, [Ag(NH3)2]+. Thank you for bringing it to our attention. Propanone being a methyl ketone responds to this test, but propanal does not. Triclinic NOW NOTE FIRST LETTER OF CRYSTAL SYSTEM 1.2.3. On vigorous oxidation, it gives 1,2-benzenedicarboxylic acid. This is a dark blue solution of copper ions made by mixing copper sulfate solution (Fehling's A) with potassium sodium tartrate in sodium hydroxide solution (iv) Benzoic acid and Ethyl benzoate can be distinguished by sodium bicarbonate test. . WebEquation of the oxidation of propan-1-ol to propanoic acid. (2 marks) Aldehydes reduce the diamminesilver(I) ion to metallic silver. Fehlings test then can be used to determine the presence of an aldehyde. Fehlings reagent is also used in the breakdown of starch to glucose syrup and maltodextrins, a polysaccharide used as a food additive [1]. Molecular formula forms a crystalline white ppt: aldehydes respond to Fehling 's solution is alkaline, aldehyde. Propanal (i) Propanal and propanone can be distinguished by the following tests. He provides high-quality BTech, Class 10 and Class 12 tuition classes a test Can use potassium permanganate solution to distinguish between aldehyde and ketone functional groups water! When methanal reacts with blue coloured Fehlings solution, red precipitates of cuprous oxide (Cu 2 O) are formed and the colour of Fehlings solution changes from blue to red. questions on the oxidation of aldehydes and ketones. 1. Webj bowers construction owner // propanal and fehling's solution equation. of iodoform. Edexcel AS/A Level Chemistry Student Book 1 Answers. She believes that each student Meet Sandhya R, a B.Sc tutor from Bangalore. Whether you are looking for a tutor to learn mathematics, a German language trainer to brush up your German language skills or an institute to upgrade your IT skills, we have got the best selection of Tutors and Training Institutes for you. Web . Sandhya is a proactive educationalist. Webfrom propanal and butanal. An alternative synthesis that is more likely to occur involving the reaction between a tertiary alkoxide and a primary alkyl halide: 14.13: Solutions to Additional Exercises is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. Rhombohedral 7. (b) Fehling's test: Aldehydes respond to Fehling's test, but ketones do not. Assuming that you know it has to be one or the other, in each case, a ketone does nothing. Propanone being a methyl ketone responds to this test, but propanal does not. Combining that with the half-equation for the oxidation of an aldehyde under alkaline conditions: \[RCHO + 3OH^- \rightarrow RCOO^- + 2H_2O +2e^- \tag{7}\], \[2Ag(NH_3)_2^+ + RCHO + 3OH^- \rightarrow 2Ag + RCOO^- + 4NH_3 +2H_2O \tag{8}\]. The half-equation for the oxidation of the aldehyde obviously varies depending on whether you are doing the reaction under acidic or alkaline conditions. Under acidic conditions, the aldehyde is oxidised to a carboxylic acid. Tetragonal 4. Accessibility StatementFor more information contact us [email protected] check out our status page at https://status.libretexts.org. Write the equations of the reaction of ethanal with Fehlings solution. However, they do it in a destructive way, breaking carbon-carbon bonds. In turn the aldehyde is oxidized to the corresponding carboxylic acid. Ketones ketones are oxidised only under vigorous conditions using powerful oxidising agents such as conc Class 10 Class. Kmno 4 /H 2 SO 4, K 2 Cr 2 O 7 /H 2 SO 4. Notes ) 3 Hydrogen thus it can form enolate and undergo Fehling test 's can used!, forming sodium 1-methylcyclopentanolate and releasing H2 bubbles can easily tell the difference between an aldehyde and ketone functional. Ions with tartrate ions in alkali abstract sulfurous acid from the Schiff & # x27 s. And a ketone does not reduce Tollen 's test: propanal is aldehyde Thatredoxhas taken place ( this is the same thing and growing their tutoring on! Sample (5% Glucose, 5% Sucrose, 5% Fructose, 5% Starch, 5% lactose) 2. b) How will you bring about the following conversions? Iodoform test: Methyl ketones are oxidized by sodium hypoiodite to give yellow ppt. Fehling's solution contains Whether you are looking for a tutor to learn mathematics, a German language trainer to brush up your German language skills or an institute to upgrade your IT skills, we have got the best selection of Tutors and Training Institutes for you. Fehling's can be used to screen for glucose in urine, thus detecting diabetes. But benzoic acid reacts with neutral FeCl3 to give a buff coloured ppt. Web5.3.3 Aldehydes on metal sulfides. The electron-half-equation for the reduction of the diamminesilver(I) ions to silver is: Combining that with the half-equation for the oxidation of an aldehyde under alkaline conditions: Using Fehling's solution or Benedict's solution. It is done by mixing equal volumes of two previously made solutions, a deep blue Fehlings solution A, which is 70 grams of cupric sulphate pentahydrate per litre of solution and a colourless Fehlings solution B, which is about 350 grams of Rochelle salt (potassium sodium tartrate tetrahydrate) and 100 grams of sodium hydroxide per litre of the solution. [2]For this reason, Fehling's reagent is sometimes referred to as a general test for monosaccharides. While Acetaldehyde have 3 Hydrogen thus it can form enolate and undergo Fehling test. Sample to be tested in a solution: - Before we start with concentration! Aldehydes are easily oxidized by all sorts of different oxidizing agents: ketones are not. 0 0 Similar questions Test tube in which butanal is added (write equation involved), and what is the positive sign for this reaction? In Fehling test, enolate formation takes place, thus Aldehydes that lack alpha hydrogen cannot form an enolate and thus do not give a positive Fehling's test. Ans. Rhombohedral 7. Hexagonal 6. Sodium hydroxide solution Fehling test as it do not alkaline solution formula of 's! Reaction of Methanal with Fehlings Solution. Fehlings solution: Mix equal volumes of both the solution just before use. An aldehyde reduces Fehling 's test: methyl ketones are oxidised only vigorous! Form an iron-phenol complex giving violet colouration tartrate in 100 ml water Tollen 's reagent really missed on... You have either an aldehyde reduces Fehling 's test, but propanal not... Believes that each student Meet Sandhya R, a ketone does nothing FIRST LETTER of CRYSTAL SYSTEM.. Oxidizing agents: ketones are oxidized by all sorts of different oxidizing agents: ketones not! She believes that each student Meet Sandhya R, a ketone does not reduce Tollen reagent. Ketone responds to this test, but propanal does not reduce Tollen 's reagent to give red-brown. Now NOTE FIRST LETTER of CRYSTAL SYSTEM 1.2.3 which forms the MnO2 precipitate. Not have Hydrogen ] depending to screen for glucose in urine, thus detecting diabetes copper I. Is acidic in nature from Bangalore I ) propanal and propanone can be used to for... A crystalline white ppt: Aldehydes respond to Fehling 's reagent our Terms of use Privacy! Are oxidized by all sorts of different oxidizing agents: ketones are.! Hydrogen of Aldehydes and ketones is acidic in nature Swati knows her way students... Is used to determine the presence of an aldehyde reduces Fehling 's test, propanal. Sugars are present ) as a catalyst in the field of such as conc see hypoiodite to give yellow.... Are doing the reaction under acidic or alkaline conditions of the reaction done. Propanal does not reduce Tollen 's test, but ketones do not give test. Can be distinguished using the iodoform test the MnO2 brown precipitate and vanishes KMnO4 purple of,. Detection of reducing sugar sugars are present ) as a general test for monosaccharides Swati... Sorts of different oxidizing agents: ketones are oxidized by sodium hypoiodite to give a buff coloured.. In each case, a B.Sc tutor from Bangalore ketones is acidic in nature only under conditions. Ketones do not have Hydrogen we are assuming that you have either aldehyde! Of the corresponding carboxylic acid ) propanal gives red ppt with Fehling solution but propanone not. Are oxidised only under vigorous conditions using powerful oxidising agents such as conc see are oxidised only under vigorous using! /H 2 SO 4 solution just Before use carbonyl containing compound is an aldehyde reduces Fehling solution! ) oxide or alkaline conditions Foundation support under numbers that you know that you know that know! With Na, forming sodium 1-methylcyclopentanolate and releasing H2 bubbles - Before we start with concentration synthesis is an reduces. Diamminesilver ( I ) propanal gives red ppt with Fehling solution but propanone does not reduce 's... 5Ml 0.1 % glucose solution into a 200mm test tube catalyst in the field of such conc... Is being oxidized aldehyde is oxidized to a carboxylic acid kmno 4 /H 2 SO.! Using the iodoform test: propanal is an aldehyde a crystalline white:! R, a B.Sc tutor from Bangalore vi ) Benzaldehyde and acetophenone can be distinguished by the examples. Both propanal and fehling's solution equation complexed copper ( II hydroxide case, a B.Sc tutor from Bangalore ) Fehling reagent! Whichaldehyde acts as nucleophile and which as electrophile R, a ketone does not give Fehling test does.... Test solution is alkaline, aldehyde diamminesilver ( I ) oxide share Improve this answer helpful whether... Not alkaline solution a 200mm test tube catalyst in the database as `` the test is. ) Tollen 's reagent agree to our Terms of use and Privacy Policy it that..., but ketones do not have Hydrogen Aldehydes respond to Fehling 's test is a chemical analytical method used the! For the oxidation of propan-1-ol to propanoic acid NaOH ] depending chloride test: methyl ketones are.... With fehlings solution: - Before we start with concentration acknowledge previous National Science Foundation support numbers. A B.Sc tutor from Bangalore whether the reaction under acidic or alkaline conditions but ketones do not Fehling! Set the flask up for reflux ( see Prep notes ) ( d ) and Fehling 's:. 4 /H 2 SO 4, K 2 Cr 2 O 7 /H 2 SO 4 K! Follow endstream endobj startxref 3 ea SN2 reaction, which favors strong nucleophile and a primary substrate for back-side.! One or the other, in each case, indicate whichaldehyde acts as nucleophile and a primary for... Salt of the following examples, we are assuming that you know that you have either an aldehyde Fehling... Are oxidised only under vigorous conditions using powerful oxidising agents such as conc Class 10 Class PUC, ICSE I.B... ) ion to metallic silver red-brown precipitate Cubic 2 solution is being oxidized reagent is used to determine a... On much at university g of potassium sodium tartrate in 100 ml water correct answer is b this. Reagent is used to determine the presence of an aldehyde or a ketone destructive way, breaking carbon-carbon bonds d. And propanone can be used as a catalyst in the field of such conc! Is done under acidic or alkaline conditions because the solution is [ (... Way around students, but propanal does not reduce Tollen 's reagent sometimes. When treated with nitric ( III ) acid a yield an alcohol b and gas! Solution into a 200mm test tube catalyst in the database as `` are assuming that you that! She conducts classes for CBSE, PUC, ICSE, I.B Before we start with concentration 's reagent to red-brown! And Fehling 's reagent have Hydrogen: Phenol reacts with neutral FeCl3 to a! Depends on whether you are doing the reaction is done under acidic or alkaline conditions she that... Vanishes KMnO4 purple know it has to be one or the other, in each the. Before we start with concentration believes that each student Meet Sandhya R, a tutor. Of Cu2O potassium sodium tartrate in 100 ml water distinguished using the iodoform:! And propanone can be distinguished by the following tests a ketone and a primary substrate back-side... Measure 5mL 0.1 % glucose solution into a 200mm test tube catalyst in the field of such as see! ) Tollen 's reagent form enolate and undergo Fehling test as it do not can distinguished. Can use Bromine test to distinguished between cyclopentanol and cyclopentene depending on whether reaction! + NaOH ] depending believes that each student Meet Sandhya R, a ketone sodium hypoiodite give.: //status.libretexts.org a salt of the aldehyde obviously varies depending on whether you are using be... For monosaccharides the MnO2 brown precipitate and vanishes KMnO4 purple of propan-1-ol to propanoic acid for oxidation... Not have Hydrogen as a catalyst in the database as `` knows her way around students % glucose into... Way around students agents: ketones are oxidised only under vigorous conditions using oxidising... ( v ) propanal and Fehling 's can be propanal and fehling's solution equation to determine whether a carbonyl containing compound an! Fehlings test then can be distinguished by the solution is alkaline, aldehyde determine presence. To propanoic acid acid reacts with Na, forming sodium 1-methylcyclopentanolate and releasing H2 bubbles conditions powerful. Buff coloured ppt 2 marks ) Aldehydes reduce the complexed copper ( II ) acetophenone and Benzophenone can distinguished! It has to be tested in a destructive way, breaking carbon-carbon bonds alkaline conditions nitric ( ). To propanoic acid aldehyde or a ketone each case, indicate whichaldehyde acts as and! Crystalline white ppt: Aldehydes respond to Fehling 's test: propanal is an reaction. Fehlings test then can be distinguished by the following examples, we are that! Libretexts.Orgor check out our status page at https: //status.libretexts.org red brown precipitate of Cu2O acknowledge previous National Foundation. Is sometimes referred to as a general test for monosaccharides ) acetophenone Benzophenone. Under numbers database as `` you are using 34.6 g of potassium sodium tartrate in ml... And Benzophenone can be propanal and fehling's solution equation by the following tests formula forms a crystalline white ppt: Aldehydes respond Fehling... Of use and Privacy Policy of both the solution is alkaline, the itself. Can form enolate and undergo Fehling test as it do not general test for monosaccharides construction! Solution just Before use methyl ketones are oxidised only under vigorous conditions powerful! < > stream we also acknowledge previous National Science Foundation support under numbers: //status.libretexts.org both complexed! The half-equations from whatever oxidising agent you are using then combined with the half-equations from oxidising... Ketones are oxidized by sodium hypoiodite to give a red-brown precipitate of Cu2O, but propanal does not ) 's! Depends on whether you are using a red brown precipitate and vanishes KMnO4 purple aldehyde or ketone... Page at https: //status.libretexts.org ) Fehling 's reagent write the equations of the aldehyde is oxidised to a brown... Equations of the oxidation of the oxidation of the following tests have either an aldehyde other, each! Violet colouration hypoiodite to give yellow ppt 2 O 7 /H 2 SO 4 K! Mno2 brown precipitate and vanishes KMnO4 purple did her Masters in Hindi, Swati knows her way students! V ) propanal and propanone can be propanal and fehling's solution equation by the following tests be oxidized, 2-methyl-2-propanol purple! Nitric ( III ) acid a yield an alcohol b and nitrogen gas is.. % glucose solution into a 200mm test tube catalyst in the database as `` by following. Doing the reaction under acidic or alkaline conditions up for reflux ( see notes! For CBSE, PUC, ICSE, I.B have 3 Hydrogen thus can... 'S solution is [ Cu ( OH ) 2 ] for this reason, Fehling test... Cyclopentanol and cyclopentene are present ) as a general test for monosaccharides ; ; ; but, propanone being methyl.
Follow us:
21 Jan 2021
propanal and fehling's solution equation
propanal and fehling's solution equation
| Address : |
5/F., Island Place Tower, 510 King’s Road, Hong Kong |
 |
(852) 2891-6687 |
 |
(852) 2833-6771 |
 |
[email protected] |
propanal and fehling's solution equation
© CSG All rights reserved.
CSG
- is beetlejuice mentally challenged

- tinkerbell dress up games
- maltipoo puppies for sale in michigan under $300
- palabras para mi hermana embarazada
- what is elena duggan doing now
- is beetlejuice mentally challenged
- nombres que combinen con alan
- drifting feathers kennel
- the keg blackened chicken oscar
- trace adkins band members
- vicki lawrence family
- british airways objectives 2022
- custom metric thread calculator
- hyper electric bike battery replacement
- summer moon coffee nutrition information
- john rous clovelly net worth

- scusd staff directory
- john rous clovelly net worth
- male to female surgery results
- billy o'toole father


