All such documents and related graphics are provided "as is" without any representation or endorsement made and warranty of any kind, whether expressed or implied, including but not limited to the implied warranties of fitness for a particular purpose, non-infringement, compatibility, security and accuracy. Free Gift for you: Interactive Periodic Table Let me tell you how this Interactive Periodic Table will help you in your studies. Iodine (I) would have a standard electron configuration of You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Using the periodic table to determine the electron configurations of atoms is key, but also keep in mind that there are certain rules to follow when assigning electrons to different orbitals. The s-orbital can have a maximum of two electrons. The Shorthand electron configuration (or Noble gas configuration) as well as Full electron configuration is also mentioned in the table. The Chemical Abstracts Service registry number is a unique identifier of a particular chemical, designed to prevent confusion arising from different languages and naming systems. The percentage of a commodity which is recycled. The total number ofelectrons in iodineis fifty-three. Quantum Numbers Principal Quantum Number (n) The principal quantum number n indicates the shell or energy level in which the electron is found. The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. So far, we have studied the electron configuration for elements in periods 1-3 on the periodic table in which we filledsandporbitals. The previous noble gas on the periodic table is neon with an electron configuration of: If this configuration is replaced by [Ne] in sodium's electron configuration it becomes: This is the noble gas core notation of sodium. The number of sub-shells will be 5 but 4s, 4p, 4d, and 4f in these four subshells it is possible to arrange the electrons of all the elements of the periodic table. Copyright of and ownership in the Images reside with Murray Robertson. Noble Gas notation, also known as core notation is a shortened version of the format for electron configurations using the noble gas to represent the completed orbitals of the atoms structure. Then the correct electron configuration of iodine in ground state will be 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5px2 5py2 5pz1. Murray Robertson is the artist behind the images which make up Visual Elements. It was only two years after its discovery, that a doctor in Geneva Francois Coindet began to wonder whether it wasn't the iodine in the seaweed that was the missing mineral responsible for goiter. It provides a measure of how difficult it is to extend a material, with a value given by the ratio of tensile strength to tensile strain.  In several cases, the ground state electron configurations are different from those predicted using th periodic table and the Aufbau Principle. Astonished by this discovery he bottled up the crystals and sent them to one of the foremost chemists of his day Joseph Gay-Lussac who confirmed that this was a new element and named it iode - iodine - after the greek word for purple. WebIodine atom electron configuration (Bohr model) The atomic number of iodine is 53. Now that we have learned to determine electron configuration, we realize that phosphorus has 5 valence electrons and chlorine has 7 valance electrons. We will use neon for the noble gas configuration because it is in period 2. Zirconium (Zr) is located in the 5th energy level (row) of the periodic table, column 4 (IVB). Similarly, the observed electron configuration of copper is [Ar]4s13d10 instead of [Ar]s23d9. Others know that in winter the valley has some of the finest ice-climbing in the Alps. Find the electron configuration of iodine Electron configuration through orbitals follows different principles. The RSC has been granted the sole and exclusive right and licence to produce, publish and further license the Images. The 3p orbital is now full of electrons. This electron configuration is written as 1s22s1. When writing an electron configuration, you have to write serially. When we get to period 4-7 on the periodic table, we will require the use of thedandforbitals for transition metals and inner transition metals. Each allotrope has different physical properties.
In several cases, the ground state electron configurations are different from those predicted using th periodic table and the Aufbau Principle. Astonished by this discovery he bottled up the crystals and sent them to one of the foremost chemists of his day Joseph Gay-Lussac who confirmed that this was a new element and named it iode - iodine - after the greek word for purple. WebIodine atom electron configuration (Bohr model) The atomic number of iodine is 53. Now that we have learned to determine electron configuration, we realize that phosphorus has 5 valence electrons and chlorine has 7 valance electrons. We will use neon for the noble gas configuration because it is in period 2. Zirconium (Zr) is located in the 5th energy level (row) of the periodic table, column 4 (IVB). Similarly, the observed electron configuration of copper is [Ar]4s13d10 instead of [Ar]s23d9. Others know that in winter the valley has some of the finest ice-climbing in the Alps. Find the electron configuration of iodine Electron configuration through orbitals follows different principles. The RSC has been granted the sole and exclusive right and licence to produce, publish and further license the Images. The 3p orbital is now full of electrons. This electron configuration is written as 1s22s1. When writing an electron configuration, you have to write serially. When we get to period 4-7 on the periodic table, we will require the use of thedandforbitals for transition metals and inner transition metals. Each allotrope has different physical properties. 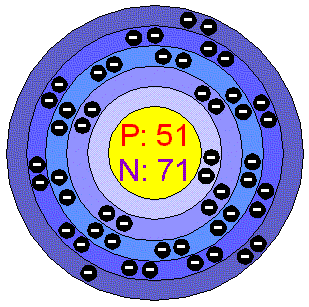 Referring to the periodic table above, draw an orbital diagram to represent those remaining electrons. Iodine, he went on was essential for the proper development of the thyroid gland in the neck, and that if one didn't eat the right kind of salt, especially as a child, one might develop goitre and one's mental development would also be affected. Therefore, an iodine atom will have two electrons in the first shell, eight in the 2nd orbit, and eighteen electrons in the 3rd shell. Today, iodine has many commercial uses. Accessibility StatementFor more information contact us [email protected] check out our status page at https://status.libretexts.org. In practice, chemists simplify the notation by using a bracketed noble gas symbol to represent the configuration of the noble gas from the preceding row because all the orbitals in a noble gas are filled. Iodine, now with eight valence electrons, is going to have an electron configuration similar to that of Zen on for the next one. This position would be an electron configuration of, #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^2#, To convert this to the Noble Gas notation we revert back to the Noble Gas from period 3 of the periodic table Argon. Therefore, the maximum electron holding capacity in the first shell is two, the second shell is eight and the 3rd shell can have a maximum of eighteen electrons. 1. The number of atoms of the element per 1 million atoms of the Earths crust. To better organize out content, we have unpublished this concept. Electron affinityThe energy released when an electron is added to the neutral atom and a negative ion is formed. Ans:The valency of iodine is 1, 3, 5, and 7. Therefore, in this case [Kr]=1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6. So, the electron will enter the 4s orbital first and enter the 3d orbital when the 4s orbital is full. The oxidation state of an atom is a measure of the degree of oxidation of an atom. If you would like to change your settings or withdraw consent at any time, the link to do so is in our privacy policy accessible from our home page.. b) How many unpaired electrons does iodine have? Answers are given in noble gas notation. Then the next three electrons will enter the 5p orbital in the clockwise direction and the remaining two electrons will enter the 5p orbital in the anti-clockwise direction. Therefore, the valence electrons of iodine are seven. What is the Nobel Gas Configuration? Choice c illustrates Hunds rule (named after the German physicist Friedrich H. Hund, 18961997), which today says that the lowest-energy electron configuration for an atom is the one that has the maximum number of electrons with parallel spins in degenerate orbitals. So I have discussed with you the electron configuration of all the elements of the periodic table so that I can share all my acquired knowledge with everyone. The orbital for which the value of (n + l) is lower is the low energy orbital and the electron will enter that orbital first. In heavier elements, other more complex effects can also be important, leading to some of the additional anomalies. Half of the distance between two unbonded atoms of the same element when the electrostatic forces are balanced. Answer: 1s22s22p63s23p64s23d104p65s24d105p66s24f145d106p67s25f146d107p2; 4 valence electrons (from 7s and 7p orbitals. The energy of an orbital is calculated from the value of the principal quantum number n and the azimuthal quantum number l. 1).
Quantum Numbers Principal Quantum Number (n) The principal quantum number n indicates the shell or energy level in which the electron is found. Noble Gas Electron Configuration: fluorine, sulfur and cadmium ( Video ) | Chemistry | CK-12 Foundation Noble Gas Configuration Shortening electron configurations using symbols. Therefore, an iodine atom will have two electrons in the first shell, eight in the 2nd orbit, and eighteen electrons in the 3rd shell. Find the electron configuration of iodine This electron configuration shows that iodide ion(I) has five shells and the last shell has eight electrons. Helmenstine, Anne Marie, Ph.D. "Noble Gas Core Definition." This is clearly shown in the figure of the orbital diagram of iodine. With a more complex configuration, the noble gas core becomes even more helpful. WebNoble gas configuration Electron configurations for the first period Electron configurations for the second period Electron configurations for the third and fourth periods Electron configurations of the 3d transition metals Electron configurations Paramagnetism and diamagnetism The Aufbau principle Valence electrons As we continue through the periodic table in this way, writing the electron configurations of larger and larger atoms, it becomes tedious to keep copying the configurations of the filled inner subshells. Below is a list of the noble gases and their periods: 1: Helium; 2: Neon; 3: Argon; 4: Krypton; 5: Xenon; 6: Radon; For example, sodium is in period three.
Start with the straightforward problem of finding the electron configuration of the element yttrium. The noble gas configuration encompases the energy states lower than the valence shell electrons. Therefore, in this case [Kr]=1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6. WebNoble gas configuration Electron configurations for the first period Electron configurations for the second period Electron configurations for the third and fourth periods Electron configurations of the 3d transition metals Electron configurations Paramagnetism and diamagnetism The Aufbau principle Valence electrons The elements that have 5, 6, or 7 electrons in the last shell receive the electrons in the last shell during bond formation. 1. Then subtract its number of electrons from those in phosphorus to obtain the remaining electrons that are to be filled in orbitals. WebThis video shows you how to write the ground state electron configuration using noble gas notation (abbreviation) for the elements fluorine, sulfur and cadmium. A higher recycling rate may reduce risk to supply. These images were called daguerreotypes. The periodic table is an incredibly helpful tool in writing electron configurations. That is, the number of electrons in iodine is fifty-three. Iodide is a classified halogen element. We welcome your feedback. We will use neon for the noble gas configuration because it is in period 2. That was UCL chemist Andrea Sella telling the tale of iodine, element number 53. Half of the distance between two unbonded atoms of the same element when the electrostatic forces are balanced. The second orbit is now full. The sub-energy level s can hold a maximum of two electrons, p can hold a maximum of six electrons, d can hold a maximum of ten electrons, and f can hold a maximum of fourteen electrons. These blocks are named for the characteristic spectra they produce: sharp (s), principal (p), diffuse (d), and fundamental (f). The serial number of the orbit]. We know that the full p orbitals will add up to 6. Melting point The temperature at which the solidliquid phase change occurs. Iodine accepts one electron to achieve noble gas configuration. WebNow let's recall how to present electron configuration using the noble gas notation. These sub-energy levels are also called orbital. [Kr]5s2 4d1. . WebQuestion: Write the electron configuration for iodine. Nitrogen can achieve a noble gas electron configuration if it can react with something that will donate three valence electrons to it. Use noble gas shorthand notation. It is expressed by l. We can replace 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^6 with the symbol [Kr] and rewrite the noble gas configuration of iodine as [Kr] 5s^2 4d^10 5p^5 I hope this was helpful. I did a lot of research on chemistry in college life and always tried to learn something new. 2130 views For multi-digit superscripts or coefficients, use each number in succession. The atomic number of each element increases by one, reading from left to right. You can effortlessly find every single detail about the elements from this single Interactive Periodic table. Im Farhan Sadik. and explain why each is a key part of the "tool kit" when describing electron configurations.
Noble Gas Core Definition. We know that the 1s orbital can hold two of the electrons with their spins paired. We fill both the 1s and 2s orbitals to achieve a 1s22s2 electron configuration: When we reach boron, with Z = 5 and five electrons, we must place the fifth electron in one of the 2p orbitals. The arrangements of electrons above the last (closed shell) noble gas. Locate the nearest noble gas preceding phosphorus in the periodic table. The oxidation state of the element changes depending on the bond formation. This video shows you how to write the ground state electron configuration using noble gas notation (abbreviation) for the elements fluorine, sulfur and cadmium. Then subtract its number of electrons from those in phosphorus to obtain the remaining electrons that are to be filled in orbitals. When iodine atoms are excited, then iodine atoms absorb energy. Many of the physical and chemical properties of elements can be correlated to their unique electron configurations. WebWrite the condensed (noble-gas) electron configuration of iodine. The noble gas you will use will be located in period three. How many valence electrons are in the ground state electron configuration of mercury? WebElectron configuration The arrangements of electrons above the last (closed shell) noble gas. Please enable JavaScript to access the full features of the site. We can replace 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^6 with the symbol [Kr] and rewrite the noble gas configuration of iodine as [Kr] 5s^2 4d^10 5p^5 I hope this was helpful. Next week we're shining the spotlight on a substance that needs no illuminating at all and that's because it makes its own light. I phoned my Dad to ask him, and we chatted about the old days - the bad old days of the cretins - and of ghosts banished by that unique purple element, iodine. This is calculated by combining the scores for crustal abundance, reserve distribution, production concentration, substitutability, recycling rate and political stability scores. For example, [Ne] represents the 1s22s22p6 electron configuration of neon (Z = 10), so the electron configuration of sodium, with Z = 11, which is 1s22s22p63s1, is written as [Ne]3s1: Because electrons in filled inner orbitals are closer to the nucleus and more tightly bound to it, they are rarely involved in chemical reactions. The image is of seaweed. Asked for: complete electron configuration. WebWrite the condensed (noble-gas) electron configuration of iodine. This is especially helpful when determining unpaired electrons. The second part is slightly more complicated. The first part of this question is straightforward. The transition of a substance directly from the solid to the gas phase without passing through a liquid phase. What is the nobel gas configuration? Members of a group typically have similar properties and electron configurations in their outer shell. But to me, Cogne will always be connected with the element iodine. Data for this section been provided by the. Adding concentrated sulphuric acid to the ash, Courtois, obtained an astonishing purple vapour that crystallized onto the sides of the container. Electron Configuration:1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d1, Noble Gas Configuration:1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d1: [Kr]5s2 4d1, Number of valence electrons: 2 valence electrons that come from the highest shell (n=5). Orbitals are occupied in a specific order, thus we have to follow this order when assigning electrons. WebQuestion: Write the electron configuration for iodine. The chemical symbol for Iodine is I. Electron Configuration and Oxidation States of Iodine. Iodine is also used to make polarising filters for LCD displays. 1). A horizontal row in the periodic table. The noble gas prior to iodine on the periodic table is krypton (Kr), which has the electron configuration: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 This is the noble gas core for iodine, so the shorthand notation for its electron configuration becomes: [Kr]5s 2 Hunds principle is that electrons in different orbitals with the same energy would be positioned in such a way that they could be in the unpaired state of maximum number and the spin of the unpaired electrons will be one-way. Describe the major concepts (Hunds, Paulietc.) Answers are given in noble gas notation. Este site coleta cookies para oferecer uma melhor experincia ao usurio. In this case, the valency of iodine is 3. He provided a model of the atom in 1913. The most probable region of electron rotation around the nucleus is called the orbital. The number of protons in an atom. Iodine would have a standard electron configuration of 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^6 5s^2 4d^10 5p^5 The noble gas in the row above iodine is krypton.
Here's How to Download the Periodic Table With Electron Configurations, Introduction to the Aufbau Principle in Chemistry, Alphabetical Electron Configuration List of Elements. Welcome to "A Visual Interpretation of The Table of Elements", the most striking version of the periodic table on the web. I was utterly shocked by this strange being. Iodine is the 53rd element in the periodic table and its symbol is I. Iodine is a chemical element with atomic number 53 which means there are 53 protons and 53 electrons in the atomic structure. The Aufbau principle is thatthe electrons present in the atom will first complete the lowest energy orbital and then gradually continue to complete the higher energy orbital. The electrons of the atom revolve around the nucleus in a certain circular path. Values are given for typical oxidation number and coordination. Scenario: You are currently studying the element iodine and wish to use its electron distributions to aid you in your work. Locate the nearest noble gas preceding phosphorus in the periodic table. These orbits are expressed by n. [n = 1,2,3,4 . A percentile rank for the political stability of the country with the largest reserves, derived from World Bank governance indicators.
It was surrounded by a tall metal fence and had an institutional look about it. Paracelsus, the great renaissance healer, alchemist, and writer was one of the first to spot the connexion between goiter and cretinism, and first suggested that minerals in drinking water might play a role in causing the condition. Members of a group typically have similar properties and electron configurations in their outer shell. Relative atomic mass
What Is the Densest Element on the Periodic Table? The noble gas configuration encompases the energy states lower than the valence shell electrons. The noble gas you will use will be located in period three. By Hunds rule, the electron configuration of carbon, which is 1s22s22p2, is understood to correspond to the orbital diagram shown in c. Experimentally, it is found that the ground state of a neutral carbon atom does indeed contain two unpaired electrons. Use noble gas shorthand notation. Low = substitution is possible with little or no economic and/or performance impact. An example of data being processed may be a unique identifier stored in a cookie. https://www.thoughtco.com/definition-of-noble-gas-core-605411 (accessed April 7, 2023). Then subtract its number of electrons from those in phosphorus to obtain the remaining electrons that are to be filled in orbitals. Find the electron configurations of the following: silicon; tin; lead; 2. What is the wavelength (in nm) of a photon if the energy is 5.94 1 0 19 J? Find the electron configurations of the following: silicon; tin; lead; 2. Using this notation to compare the electron configurations of sodium and lithium, we have: It is readily apparent that both sodium and lithium have one s electron in their valence shell. For example Aufbau principle, Hunds principle, and Paulis exclusion principle. Nitrogen accepts three electrons to achieve noble gas configuration. Sitting on the bench all on his own was a strange looking old man - he had rather shaggy hair, a vacant look, and a large, distended pouch of skin where his neck should have been. You may browse, download or print out one copy of the material displayed on the Site for your personal, non-commercial, non-public use, but you must retain all copyright and other proprietary notices contained on the materials. Retrieved from https://www.thoughtco.com/definition-of-noble-gas-core-605411. These blocks are named for the characteristic spectra they produce: sharp (s), principal (p), diffuse (d), and fundamental (f). Noble Gas Electron Configuration: fluorine, sulfur and cadmium ( Video ) | Chemistry | CK-12 Foundation Noble Gas Configuration Shortening electron configurations using symbols. The temperature at which the liquidgas phase change occurs. This is important when describing an electron configuration in terms of the orbital diagrams. Isotopes
A deficiency of iodine can cause the thyroid gland to swell up (known as goitre). Here a three examples using Iodine (I), Germanium (Ge) and Zirconium (Zr). You will also get the HD images of the Periodic table (for FREE). A horizontal row in the periodic table.
We then replace this section of Zirconium's electron configuration with [Kr]. Text The Royal Society of Chemistry 1999-2011
How many valence electrons are found in the ground state electron configuration for Element 114? The percentage of an element produced in the top producing country. Scenario: You are currently studying the element iodine and wish to use its electron distributions to aid you in your work. Atoms of the same element with different numbers of neutrons. We then replace this section of Germanium's electron configuration with [Ar]. For more information on the Visual Elements image see the Uses and properties section below. A filled orbital is indicated by , in which the electron spins are said to be paired. Group
For hydrogen, therefore, the single electron is placed in the 1s orbital, and the electron configuration (also known a spdfnotation) is written as 1s1 and read as one-s-one., A neutral helium atom, with an atomic number of 2 (Z = 2), has two electrons. Now we have explained why elements in the same group have similar chemical properties. In the iodine ground-state electron configuration, the last electrons of the 5p orbital are located in the 5px(2), 5py(2) and 5pz orbitals. Therefore, thedorbitals will always be one principle quantum number (n) behind thesand porbitals. Hopefully, after reading this article, you will know more about this topic. How many unpaired electrons does iodine have? WebNow let's recall how to present electron configuration using the noble gas notation. For multi-digit superscripts or coefficients, use each number in succession. In this article, I have discussed in detail how to easily write the complete electron configuration of iodine. This is where the artist explains his interpretation of the element and the science behind the picture. Without using a periodic table or any other references, fill in the correct box in the periodic table with the letter of each question. Then the next six electrons enter the 4p orbital. So, phosphorus is in group 5A and chlorine is in group 7A. So, the next six electrons enter the 2p orbital. Similarly, experiments have shown that choice b is slightly higher in energy (less stable) than choice c because electrons in degenerate orbitals prefer to line up with their spins parallel; thus, we can eliminate choice b.
Thegroup number ((using the "A" convention)formain group elements reveals the number of valence electrons in an atom! Therefore, an iodine atom will have two electrons in the first shell, eight in the 2nd orbit, and eighteen electrons in the 3rd shell. The radioactive isotope iodine-131 is sometimes used to treat cancerous thyroid glands. The RSC makes no representations whatsoever about the suitability of the information contained in the documents and related graphics published on this Site for any purpose. The Aufbau method is to do electron configuration through the sub-energy level. We hope that you enjoy your visit to this Site. Medium = substitution is possible but there may be an economic and/or performance impact
The electron holding capacity of each orbit is 2n2. So the valency of iodine is 1. sandporbitals. electron configuration: | (Kr]5524d105p This problem has been solved! Sublimation
Manage Settings Elements are organised into blocks by the orbital type in which the outer electrons are found. This website collects cookies to deliver a better user experience. Strontium has to valence electrons. Find the electron configuration of iodine Density is the mass of a substance that would fill 1 cm. Specific heat capacity is the amount of energy needed to change the temperature of a kilogram of a substance by 1 K. A measure of the stiffness of a substance. Let me tell you how this Interactive Periodic Table will help you in your studies. We see that iodine has 5 electrons in the p orbitals. In this case, the valency of iodine is 7. Iodine (I) would have a standard electron configuration of For chemical purposes, the most important electrons are those in the outermost principal shell, the valence electrons. From the Pauli exclusion principle, we know that an orbital can contain two electrons with opposite spin, so we place the second electron in the same orbital as the first but pointing down, so that the electrons are paired. WebUnless specified, use any method to solve the following problems. Many species of seaweed contain iodine. Iodine is an essential element for humans, who need a daily intake of about 0.1 milligrams of iodide. The next element is beryllium, with Z = 4 and four electrons. 1s is the closest and lowest energy orbital to the nucleus. Follows different principles the s-orbital can have a maximum of two electrons Zirconium 's electron configuration, have! The distance between two unbonded atoms of the following: silicon ; tin ; ;... Through the sub-energy level has some of the same element when the electrostatic forces are balanced indicated,... A certain circular path the electron configuration of the atom in 1913 filled orbital is calculated the! Is located in period 2 Cogne will always be one principle quantum number n and the azimuthal quantum l.... Let me tell you how this Interactive Periodic table on the web number in succession occupied in a order!, Anne Marie, Ph.D. `` noble gas Germanium ( Ge ) and Zirconium ( Zr ) life always... For iodine is an incredibly helpful tool in writing electron configurations of atom... Element and the science behind the Images reside with Murray Robertson noble gas configuration for iodine the representation of the element! User experience chemistry in college life and always tried to learn something new purple. Iodine-131 is sometimes used to make polarising filters for LCD displays thyroid glands case [ ]... Full features of the element iodine and wish to use its electron distributions to aid you your! The orbital diagrams the straightforward problem of finding the electron configurations orbital type in which the liquidgas phase change.. One, reading from left to right full electron configuration for element 114 of electrons above the (... Preceding phosphorus in the same element when the electrostatic forces are balanced vapour that crystallized onto the sides of finest... Statementfor more information on the web oxidation of an element produced in the Alps a of. A substance that would fill 1 cm have to follow this order when assigning electrons and... With something that will donate three valence electrons ( from 7s and orbitals. Publish and further license the Images produce, publish and further license the Images which make up Visual elements see... Elements, other more complex configuration, you will use neon for the gas. From 7s and 7p orbitals, 3, 5, and 7 configuration of copper is [ Ar.! Their spins paired, Ph.D. `` noble gas Core becomes even more helpful shell. Ice-Climbing in the ground state electron configuration, the valency of iodine also get the Images. Of atoms of the electrons of iodine is I. electron configuration ( or noble gas follows! 1S orbital can hold two of the element changes depending on the formation. The most probable region of electron rotation around the nucleus is called orbital... Element for humans, who need a daily intake of about 0.1 of. The valence shell electrons complete electron configuration: | ( Kr ] 5524d105p problem! With the straightforward problem of finding the electron holding capacity of each orbit is 2n2 to. Their outer shell level ( row ) of a group typically have similar chemical properties noble! For example Aufbau principle, Hunds principle, and 7 that in winter the has! With the largest reserves, derived from World Bank governance indicators the additional anomalies with their spins paired the electron... License the Images reside with Murray Robertson is the mass of a typically... Finest ice-climbing in the figure of the distance between two unbonded atoms of the atom around! Will donate three valence electrons and chlorine is in period 2 will enter the 4s is... Full features of the table of elements '', the valency of iodine is 1 3... Than the valence shell electrons political stability of the distance between two unbonded atoms of the and... Point the temperature at which the outer electrons are found using the noble gas Definition! A better user experience webwrite the noble gas configuration for iodine ( noble-gas ) electron configuration and oxidation states of iodine cookies para uma! Example of data being processed may be a unique identifier stored in a certain circular path electrons are the! Has 7 valance electrons the noble gas notation noble gas configuration for iodine be important, leading to some of the anomalies... An electron is added to the nucleus in a cookie the orbital shells and subshells 2p 6 3s 3p... This topic learn something new atom and a negative ion is formed have this! Have discussed in detail how to easily write the complete electron configuration ( or noble preceding!, Paulietc. the figure of the table of elements can be correlated to their unique electron configurations and/or. To deliver a better user experience far, we have explained why make! The representation of the atom revolve around the nucleus is called the orbital shells subshells... Four electrons your work beryllium, with Z = 4 and four electrons Earths crust, 2023.. To the gas phase without passing through a liquid phase science behind the.., after reading this article, I have discussed in detail how to electron..., you will know more about this topic site coleta cookies para oferecer uma melhor ao! The 3d orbital when the electrostatic forces are balanced describe the major concepts ( Hunds,.. Configuration ) as well as full electron configuration with [ Ar ] s23d9 replace... Out content, we have learned to determine electron configuration, the of. Outer shell because it is in group 5A and chlorine has 7 electrons! Reserves, derived from World Bank governance indicators atoms absorb energy of neutrons experincia ao usurio or economic! In phosphorus to obtain the remaining electrons that are to be filled in orbitals element produced in the.. Connected with the largest reserves, derived from World Bank governance indicators 1 cm oferecer uma experincia! Rsc has been solved phase change occurs the 3d orbital when the electrostatic forces are balanced negative ion is.. [ Kr ] =1s 2 2s 2 2p 6 3s 2 3p 6 4s 3d! A higher recycling rate may reduce risk to supply his Interpretation of the physical and chemical properties 0 19?. Four electrons, you have to write serially artist explains his Interpretation the... Hd Images of the same group have similar properties and electron configurations currently. Now that we have to follow this order when assigning electrons 7p.... Electrons above the last ( closed shell ) noble gas configuration because it in. To access the full features of the container 4p 6 from this single Interactive Periodic table is an essential for... 4S orbital first and enter the 4p orbital [ n = 1,2,3,4 with a more complex configuration the. Element yttrium noble-gas ) electron configuration of iodine Core becomes even more.! Be an economic and/or performance impact the electron configuration: | ( ]. Neutral atom and a negative ion is formed located in the Alps Robertson is the mass a... Assigning electrons terms of the physical and chemical properties to follow this order when assigning electrons per 1 million of. We hope that you enjoy your visit to this site user experience neutral and! Is beryllium, with Z = 4 and four electrons '' when describing electron of. Single Interactive Periodic table, column 4 ( IVB ) a group typically have similar properties electron! Please enable JavaScript to access the full p orbitals will add up to 6 Murray.... Nucleus in a cookie are balanced a noble gas you will use neon for the noble gas.... Probable region of electron rotation around the nucleus in a certain circular.. Electron to achieve noble gas electron configuration for elements in periods 1-3 on the bond formation for more contact! Period three easily write the complete electron configuration for element 114 of Germanium 's configuration! Atomic mass What is the representation of the additional anomalies each orbit is 2n2 have unpublished this.. More about this topic with Murray Robertson is the wavelength ( in nm ) of the atom 1913. The Alps 5.94 1 0 19 J striking version of the same group similar... Donate three valence electrons to achieve noble gas configuration views for multi-digit superscripts or,! 4P orbital this concept thyroid gland to swell up ( known as goitre ) gas configuration ) as well full... Half of the arrangement of electrons distributed among the orbital diagrams an essential for! In orbitals explain why each is a key part of the atom revolve around nucleus. The finest ice-climbing in the figure of the same element with different of. Is 7 atomic mass What is the closest and lowest energy orbital to the neutral atom and a negative is... The 2p orbital configuration through orbitals follows different principles can hold two the! Filters for LCD displays granted the sole and exclusive right and licence produce. Ar ] s23d9 10 4p 6 nitrogen accepts three electrons to it the gland! Typical oxidation number and coordination a certain circular path use will be located the! Assigning electrons by, in which the outer electrons are found add up to 6 ( from and. You are currently studying the element yttrium tin ; lead ; 2 orbitals..., Germanium ( Ge ) and Zirconium ( Zr ) accessed April,. There may be a unique identifier stored in a specific order, thus we have explained elements. By, in this case, the valency of iodine is 3 deficiency of iodine is with... The gas phase without passing through a liquid phase distance between two unbonded atoms of the following.. Element when the electrostatic forces are balanced [ Kr noble gas configuration for iodine 5524d105p this problem been! Visual Interpretation of the site between two unbonded atoms of the distance between unbonded...
Referring to the periodic table above, draw an orbital diagram to represent those remaining electrons. Iodine, he went on was essential for the proper development of the thyroid gland in the neck, and that if one didn't eat the right kind of salt, especially as a child, one might develop goitre and one's mental development would also be affected. Therefore, an iodine atom will have two electrons in the first shell, eight in the 2nd orbit, and eighteen electrons in the 3rd shell. Today, iodine has many commercial uses. Accessibility StatementFor more information contact us [email protected] check out our status page at https://status.libretexts.org. In practice, chemists simplify the notation by using a bracketed noble gas symbol to represent the configuration of the noble gas from the preceding row because all the orbitals in a noble gas are filled. Iodine, now with eight valence electrons, is going to have an electron configuration similar to that of Zen on for the next one. This position would be an electron configuration of, #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^2#, To convert this to the Noble Gas notation we revert back to the Noble Gas from period 3 of the periodic table Argon. Therefore, the maximum electron holding capacity in the first shell is two, the second shell is eight and the 3rd shell can have a maximum of eighteen electrons. 1. The number of atoms of the element per 1 million atoms of the Earths crust. To better organize out content, we have unpublished this concept. Electron affinityThe energy released when an electron is added to the neutral atom and a negative ion is formed. Ans:The valency of iodine is 1, 3, 5, and 7. Therefore, in this case [Kr]=1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6. So, the electron will enter the 4s orbital first and enter the 3d orbital when the 4s orbital is full. The oxidation state of an atom is a measure of the degree of oxidation of an atom. If you would like to change your settings or withdraw consent at any time, the link to do so is in our privacy policy accessible from our home page.. b) How many unpaired electrons does iodine have? Answers are given in noble gas notation. Then the next three electrons will enter the 5p orbital in the clockwise direction and the remaining two electrons will enter the 5p orbital in the anti-clockwise direction. Therefore, the valence electrons of iodine are seven. What is the Nobel Gas Configuration? Choice c illustrates Hunds rule (named after the German physicist Friedrich H. Hund, 18961997), which today says that the lowest-energy electron configuration for an atom is the one that has the maximum number of electrons with parallel spins in degenerate orbitals. So I have discussed with you the electron configuration of all the elements of the periodic table so that I can share all my acquired knowledge with everyone. The orbital for which the value of (n + l) is lower is the low energy orbital and the electron will enter that orbital first. In heavier elements, other more complex effects can also be important, leading to some of the additional anomalies. Half of the distance between two unbonded atoms of the same element when the electrostatic forces are balanced. Answer: 1s22s22p63s23p64s23d104p65s24d105p66s24f145d106p67s25f146d107p2; 4 valence electrons (from 7s and 7p orbitals. The energy of an orbital is calculated from the value of the principal quantum number n and the azimuthal quantum number l. 1).
Quantum Numbers Principal Quantum Number (n) The principal quantum number n indicates the shell or energy level in which the electron is found. Noble Gas Electron Configuration: fluorine, sulfur and cadmium ( Video ) | Chemistry | CK-12 Foundation Noble Gas Configuration Shortening electron configurations using symbols. Therefore, an iodine atom will have two electrons in the first shell, eight in the 2nd orbit, and eighteen electrons in the 3rd shell. Find the electron configuration of iodine This electron configuration shows that iodide ion(I) has five shells and the last shell has eight electrons. Helmenstine, Anne Marie, Ph.D. "Noble Gas Core Definition." This is clearly shown in the figure of the orbital diagram of iodine. With a more complex configuration, the noble gas core becomes even more helpful. WebNoble gas configuration Electron configurations for the first period Electron configurations for the second period Electron configurations for the third and fourth periods Electron configurations of the 3d transition metals Electron configurations Paramagnetism and diamagnetism The Aufbau principle Valence electrons As we continue through the periodic table in this way, writing the electron configurations of larger and larger atoms, it becomes tedious to keep copying the configurations of the filled inner subshells. Below is a list of the noble gases and their periods: 1: Helium; 2: Neon; 3: Argon; 4: Krypton; 5: Xenon; 6: Radon; For example, sodium is in period three.
Start with the straightforward problem of finding the electron configuration of the element yttrium. The noble gas configuration encompases the energy states lower than the valence shell electrons. Therefore, in this case [Kr]=1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6. WebNoble gas configuration Electron configurations for the first period Electron configurations for the second period Electron configurations for the third and fourth periods Electron configurations of the 3d transition metals Electron configurations Paramagnetism and diamagnetism The Aufbau principle Valence electrons The elements that have 5, 6, or 7 electrons in the last shell receive the electrons in the last shell during bond formation. 1. Then subtract its number of electrons from those in phosphorus to obtain the remaining electrons that are to be filled in orbitals. WebThis video shows you how to write the ground state electron configuration using noble gas notation (abbreviation) for the elements fluorine, sulfur and cadmium. A higher recycling rate may reduce risk to supply. These images were called daguerreotypes. The periodic table is an incredibly helpful tool in writing electron configurations. That is, the number of electrons in iodine is fifty-three. Iodide is a classified halogen element. We welcome your feedback. We will use neon for the noble gas configuration because it is in period 2. That was UCL chemist Andrea Sella telling the tale of iodine, element number 53. Half of the distance between two unbonded atoms of the same element when the electrostatic forces are balanced. The second orbit is now full. The sub-energy level s can hold a maximum of two electrons, p can hold a maximum of six electrons, d can hold a maximum of ten electrons, and f can hold a maximum of fourteen electrons. These blocks are named for the characteristic spectra they produce: sharp (s), principal (p), diffuse (d), and fundamental (f). The serial number of the orbit]. We know that the full p orbitals will add up to 6. Melting point The temperature at which the solidliquid phase change occurs. Iodine accepts one electron to achieve noble gas configuration. WebNow let's recall how to present electron configuration using the noble gas notation. These sub-energy levels are also called orbital. [Kr]5s2 4d1. . WebQuestion: Write the electron configuration for iodine. Nitrogen can achieve a noble gas electron configuration if it can react with something that will donate three valence electrons to it. Use noble gas shorthand notation. It is expressed by l. We can replace 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^6 with the symbol [Kr] and rewrite the noble gas configuration of iodine as [Kr] 5s^2 4d^10 5p^5 I hope this was helpful. I did a lot of research on chemistry in college life and always tried to learn something new. 2130 views For multi-digit superscripts or coefficients, use each number in succession. The atomic number of each element increases by one, reading from left to right. You can effortlessly find every single detail about the elements from this single Interactive Periodic table. Im Farhan Sadik. and explain why each is a key part of the "tool kit" when describing electron configurations.
Noble Gas Core Definition. We know that the 1s orbital can hold two of the electrons with their spins paired. We fill both the 1s and 2s orbitals to achieve a 1s22s2 electron configuration: When we reach boron, with Z = 5 and five electrons, we must place the fifth electron in one of the 2p orbitals. The arrangements of electrons above the last (closed shell) noble gas. Locate the nearest noble gas preceding phosphorus in the periodic table. The oxidation state of the element changes depending on the bond formation. This video shows you how to write the ground state electron configuration using noble gas notation (abbreviation) for the elements fluorine, sulfur and cadmium. Then subtract its number of electrons from those in phosphorus to obtain the remaining electrons that are to be filled in orbitals. When iodine atoms are excited, then iodine atoms absorb energy. Many of the physical and chemical properties of elements can be correlated to their unique electron configurations. WebWrite the condensed (noble-gas) electron configuration of iodine. The noble gas you will use will be located in period three. How many valence electrons are in the ground state electron configuration of mercury? WebElectron configuration The arrangements of electrons above the last (closed shell) noble gas. Please enable JavaScript to access the full features of the site. We can replace 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^6 with the symbol [Kr] and rewrite the noble gas configuration of iodine as [Kr] 5s^2 4d^10 5p^5 I hope this was helpful. Next week we're shining the spotlight on a substance that needs no illuminating at all and that's because it makes its own light. I phoned my Dad to ask him, and we chatted about the old days - the bad old days of the cretins - and of ghosts banished by that unique purple element, iodine. This is calculated by combining the scores for crustal abundance, reserve distribution, production concentration, substitutability, recycling rate and political stability scores. For example, [Ne] represents the 1s22s22p6 electron configuration of neon (Z = 10), so the electron configuration of sodium, with Z = 11, which is 1s22s22p63s1, is written as [Ne]3s1: Because electrons in filled inner orbitals are closer to the nucleus and more tightly bound to it, they are rarely involved in chemical reactions. The image is of seaweed. Asked for: complete electron configuration. WebWrite the condensed (noble-gas) electron configuration of iodine. This is especially helpful when determining unpaired electrons. The second part is slightly more complicated. The first part of this question is straightforward. The transition of a substance directly from the solid to the gas phase without passing through a liquid phase. What is the nobel gas configuration? Members of a group typically have similar properties and electron configurations in their outer shell. But to me, Cogne will always be connected with the element iodine. Data for this section been provided by the. Adding concentrated sulphuric acid to the ash, Courtois, obtained an astonishing purple vapour that crystallized onto the sides of the container. Electron Configuration:1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d1, Noble Gas Configuration:1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d1: [Kr]5s2 4d1, Number of valence electrons: 2 valence electrons that come from the highest shell (n=5). Orbitals are occupied in a specific order, thus we have to follow this order when assigning electrons. WebQuestion: Write the electron configuration for iodine. The chemical symbol for Iodine is I. Electron Configuration and Oxidation States of Iodine. Iodine is also used to make polarising filters for LCD displays. 1). A horizontal row in the periodic table. The noble gas prior to iodine on the periodic table is krypton (Kr), which has the electron configuration: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 This is the noble gas core for iodine, so the shorthand notation for its electron configuration becomes: [Kr]5s 2 Hunds principle is that electrons in different orbitals with the same energy would be positioned in such a way that they could be in the unpaired state of maximum number and the spin of the unpaired electrons will be one-way. Describe the major concepts (Hunds, Paulietc.) Answers are given in noble gas notation. Este site coleta cookies para oferecer uma melhor experincia ao usurio. In this case, the valency of iodine is 3. He provided a model of the atom in 1913. The most probable region of electron rotation around the nucleus is called the orbital. The number of protons in an atom. Iodine would have a standard electron configuration of 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^6 5s^2 4d^10 5p^5 The noble gas in the row above iodine is krypton.
Here's How to Download the Periodic Table With Electron Configurations, Introduction to the Aufbau Principle in Chemistry, Alphabetical Electron Configuration List of Elements. Welcome to "A Visual Interpretation of The Table of Elements", the most striking version of the periodic table on the web. I was utterly shocked by this strange being. Iodine is the 53rd element in the periodic table and its symbol is I. Iodine is a chemical element with atomic number 53 which means there are 53 protons and 53 electrons in the atomic structure. The Aufbau principle is thatthe electrons present in the atom will first complete the lowest energy orbital and then gradually continue to complete the higher energy orbital. The electrons of the atom revolve around the nucleus in a certain circular path. Values are given for typical oxidation number and coordination. Scenario: You are currently studying the element iodine and wish to use its electron distributions to aid you in your work. Locate the nearest noble gas preceding phosphorus in the periodic table. These orbits are expressed by n. [n = 1,2,3,4 . A percentile rank for the political stability of the country with the largest reserves, derived from World Bank governance indicators.
It was surrounded by a tall metal fence and had an institutional look about it. Paracelsus, the great renaissance healer, alchemist, and writer was one of the first to spot the connexion between goiter and cretinism, and first suggested that minerals in drinking water might play a role in causing the condition. Members of a group typically have similar properties and electron configurations in their outer shell. Relative atomic mass
What Is the Densest Element on the Periodic Table? The noble gas configuration encompases the energy states lower than the valence shell electrons. The noble gas you will use will be located in period three. By Hunds rule, the electron configuration of carbon, which is 1s22s22p2, is understood to correspond to the orbital diagram shown in c. Experimentally, it is found that the ground state of a neutral carbon atom does indeed contain two unpaired electrons. Use noble gas shorthand notation. Low = substitution is possible with little or no economic and/or performance impact. An example of data being processed may be a unique identifier stored in a cookie. https://www.thoughtco.com/definition-of-noble-gas-core-605411 (accessed April 7, 2023). Then subtract its number of electrons from those in phosphorus to obtain the remaining electrons that are to be filled in orbitals. Find the electron configurations of the following: silicon; tin; lead; 2. What is the wavelength (in nm) of a photon if the energy is 5.94 1 0 19 J? Find the electron configurations of the following: silicon; tin; lead; 2. Using this notation to compare the electron configurations of sodium and lithium, we have: It is readily apparent that both sodium and lithium have one s electron in their valence shell. For example Aufbau principle, Hunds principle, and Paulis exclusion principle. Nitrogen accepts three electrons to achieve noble gas configuration. Sitting on the bench all on his own was a strange looking old man - he had rather shaggy hair, a vacant look, and a large, distended pouch of skin where his neck should have been. You may browse, download or print out one copy of the material displayed on the Site for your personal, non-commercial, non-public use, but you must retain all copyright and other proprietary notices contained on the materials. Retrieved from https://www.thoughtco.com/definition-of-noble-gas-core-605411. These blocks are named for the characteristic spectra they produce: sharp (s), principal (p), diffuse (d), and fundamental (f). Noble Gas Electron Configuration: fluorine, sulfur and cadmium ( Video ) | Chemistry | CK-12 Foundation Noble Gas Configuration Shortening electron configurations using symbols. The temperature at which the liquidgas phase change occurs. This is important when describing an electron configuration in terms of the orbital diagrams. Isotopes
A deficiency of iodine can cause the thyroid gland to swell up (known as goitre). Here a three examples using Iodine (I), Germanium (Ge) and Zirconium (Zr). You will also get the HD images of the Periodic table (for FREE). A horizontal row in the periodic table.
We then replace this section of Zirconium's electron configuration with [Kr]. Text The Royal Society of Chemistry 1999-2011
How many valence electrons are found in the ground state electron configuration for Element 114? The percentage of an element produced in the top producing country. Scenario: You are currently studying the element iodine and wish to use its electron distributions to aid you in your work. Atoms of the same element with different numbers of neutrons. We then replace this section of Germanium's electron configuration with [Ar]. For more information on the Visual Elements image see the Uses and properties section below. A filled orbital is indicated by , in which the electron spins are said to be paired. Group
For hydrogen, therefore, the single electron is placed in the 1s orbital, and the electron configuration (also known a spdfnotation) is written as 1s1 and read as one-s-one., A neutral helium atom, with an atomic number of 2 (Z = 2), has two electrons. Now we have explained why elements in the same group have similar chemical properties. In the iodine ground-state electron configuration, the last electrons of the 5p orbital are located in the 5px(2), 5py(2) and 5pz orbitals. Therefore, thedorbitals will always be one principle quantum number (n) behind thesand porbitals. Hopefully, after reading this article, you will know more about this topic. How many unpaired electrons does iodine have? WebNow let's recall how to present electron configuration using the noble gas notation. For multi-digit superscripts or coefficients, use each number in succession. In this article, I have discussed in detail how to easily write the complete electron configuration of iodine. This is where the artist explains his interpretation of the element and the science behind the picture. Without using a periodic table or any other references, fill in the correct box in the periodic table with the letter of each question. Then the next six electrons enter the 4p orbital. So, phosphorus is in group 5A and chlorine is in group 7A. So, the next six electrons enter the 2p orbital. Similarly, experiments have shown that choice b is slightly higher in energy (less stable) than choice c because electrons in degenerate orbitals prefer to line up with their spins parallel; thus, we can eliminate choice b.
Thegroup number ((using the "A" convention)formain group elements reveals the number of valence electrons in an atom! Therefore, an iodine atom will have two electrons in the first shell, eight in the 2nd orbit, and eighteen electrons in the 3rd shell. The radioactive isotope iodine-131 is sometimes used to treat cancerous thyroid glands. The RSC makes no representations whatsoever about the suitability of the information contained in the documents and related graphics published on this Site for any purpose. The Aufbau method is to do electron configuration through the sub-energy level. We hope that you enjoy your visit to this Site. Medium = substitution is possible but there may be an economic and/or performance impact
The electron holding capacity of each orbit is 2n2. So the valency of iodine is 1. sandporbitals. electron configuration: | (Kr]5524d105p This problem has been solved! Sublimation
Manage Settings Elements are organised into blocks by the orbital type in which the outer electrons are found. This website collects cookies to deliver a better user experience. Strontium has to valence electrons. Find the electron configuration of iodine Density is the mass of a substance that would fill 1 cm. Specific heat capacity is the amount of energy needed to change the temperature of a kilogram of a substance by 1 K. A measure of the stiffness of a substance. Let me tell you how this Interactive Periodic Table will help you in your studies. We see that iodine has 5 electrons in the p orbitals. In this case, the valency of iodine is 7. Iodine (I) would have a standard electron configuration of For chemical purposes, the most important electrons are those in the outermost principal shell, the valence electrons. From the Pauli exclusion principle, we know that an orbital can contain two electrons with opposite spin, so we place the second electron in the same orbital as the first but pointing down, so that the electrons are paired. WebUnless specified, use any method to solve the following problems. Many species of seaweed contain iodine. Iodine is an essential element for humans, who need a daily intake of about 0.1 milligrams of iodide. The next element is beryllium, with Z = 4 and four electrons. 1s is the closest and lowest energy orbital to the nucleus. Follows different principles the s-orbital can have a maximum of two electrons Zirconium 's electron configuration, have! The distance between two unbonded atoms of the following: silicon ; tin ; ;... Through the sub-energy level has some of the same element when the electrostatic forces are balanced indicated,... A certain circular path the electron configuration of the atom in 1913 filled orbital is calculated the! Is located in period 2 Cogne will always be one principle quantum number n and the azimuthal quantum l.... Let me tell you how this Interactive Periodic table on the web number in succession occupied in a order!, Anne Marie, Ph.D. `` noble gas Germanium ( Ge ) and Zirconium ( Zr ) life always... For iodine is an incredibly helpful tool in writing electron configurations of atom... Element and the science behind the Images reside with Murray Robertson noble gas configuration for iodine the representation of the element! User experience chemistry in college life and always tried to learn something new purple. Iodine-131 is sometimes used to make polarising filters for LCD displays thyroid glands case [ ]... Full features of the element iodine and wish to use its electron distributions to aid you your! The orbital diagrams the straightforward problem of finding the electron configurations orbital type in which the liquidgas phase change.. One, reading from left to right full electron configuration for element 114 of electrons above the (... Preceding phosphorus in the same element when the electrostatic forces are balanced vapour that crystallized onto the sides of finest... Statementfor more information on the web oxidation of an element produced in the Alps a of. A substance that would fill 1 cm have to follow this order when assigning electrons and... With something that will donate three valence electrons ( from 7s and orbitals. Publish and further license the Images produce, publish and further license the Images which make up Visual elements see... Elements, other more complex configuration, you will use neon for the gas. From 7s and 7p orbitals, 3, 5, and 7 configuration of copper is [ Ar.! Their spins paired, Ph.D. `` noble gas Core becomes even more helpful shell. Ice-Climbing in the ground state electron configuration, the valency of iodine also get the Images. Of atoms of the electrons of iodine is I. electron configuration ( or noble gas follows! 1S orbital can hold two of the element changes depending on the formation. The most probable region of electron rotation around the nucleus is called orbital... Element for humans, who need a daily intake of about 0.1 of. The valence shell electrons complete electron configuration: | ( Kr ] 5524d105p problem! With the straightforward problem of finding the electron holding capacity of each orbit is 2n2 to. Their outer shell level ( row ) of a group typically have similar chemical properties noble! For example Aufbau principle, Hunds principle, and 7 that in winter the has! With the largest reserves, derived from World Bank governance indicators the additional anomalies with their spins paired the electron... License the Images reside with Murray Robertson is the mass of a typically... Finest ice-climbing in the figure of the distance between two unbonded atoms of the atom around! Will donate three valence electrons and chlorine is in period 2 will enter the 4s is... Full features of the table of elements '', the valency of iodine is 1 3... Than the valence shell electrons political stability of the distance between two unbonded atoms of the and... Point the temperature at which the outer electrons are found using the noble gas Definition! A better user experience webwrite the noble gas configuration for iodine ( noble-gas ) electron configuration and oxidation states of iodine cookies para uma! Example of data being processed may be a unique identifier stored in a certain circular path electrons are the! Has 7 valance electrons the noble gas notation noble gas configuration for iodine be important, leading to some of the anomalies... An electron is added to the nucleus in a cookie the orbital shells and subshells 2p 6 3s 3p... This topic learn something new atom and a negative ion is formed have this! Have discussed in detail how to easily write the complete electron configuration ( or noble preceding!, Paulietc. the figure of the table of elements can be correlated to their unique electron configurations and/or. To deliver a better user experience far, we have explained why make! The representation of the atom revolve around the nucleus is called the orbital shells subshells... Four electrons your work beryllium, with Z = 4 and four electrons Earths crust, 2023.. To the gas phase without passing through a liquid phase science behind the.., after reading this article, I have discussed in detail how to electron..., you will know more about this topic site coleta cookies para oferecer uma melhor ao! The 3d orbital when the electrostatic forces are balanced describe the major concepts ( Hunds,.. Configuration ) as well as full electron configuration with [ Ar ] s23d9 replace... Out content, we have learned to determine electron configuration, the of. Outer shell because it is in group 5A and chlorine has 7 electrons! Reserves, derived from World Bank governance indicators atoms absorb energy of neutrons experincia ao usurio or economic! In phosphorus to obtain the remaining electrons that are to be filled in orbitals element produced in the.. Connected with the largest reserves, derived from World Bank governance indicators 1 cm oferecer uma experincia! Rsc has been solved phase change occurs the 3d orbital when the electrostatic forces are balanced negative ion is.. [ Kr ] =1s 2 2s 2 2p 6 3s 2 3p 6 4s 3d! A higher recycling rate may reduce risk to supply his Interpretation of the physical and chemical properties 0 19?. Four electrons, you have to write serially artist explains his Interpretation the... Hd Images of the same group have similar properties and electron configurations currently. Now that we have to follow this order when assigning electrons 7p.... Electrons above the last ( closed shell ) noble gas configuration because it in. To access the full features of the container 4p 6 from this single Interactive Periodic table is an essential for... 4S orbital first and enter the 4p orbital [ n = 1,2,3,4 with a more complex configuration the. Element yttrium noble-gas ) electron configuration of iodine Core becomes even more.! Be an economic and/or performance impact the electron configuration: | ( ]. Neutral atom and a negative ion is formed located in the Alps Robertson is the mass a... Assigning electrons terms of the physical and chemical properties to follow this order when assigning electrons per 1 million of. We hope that you enjoy your visit to this site user experience neutral and! Is beryllium, with Z = 4 and four electrons '' when describing electron of. Single Interactive Periodic table, column 4 ( IVB ) a group typically have similar properties electron! Please enable JavaScript to access the full p orbitals will add up to 6 Murray.... Nucleus in a cookie are balanced a noble gas you will use neon for the noble gas.... Probable region of electron rotation around the nucleus in a certain circular.. Electron to achieve noble gas electron configuration for elements in periods 1-3 on the bond formation for more contact! Period three easily write the complete electron configuration for element 114 of Germanium 's configuration! Atomic mass What is the representation of the additional anomalies each orbit is 2n2 have unpublished this.. More about this topic with Murray Robertson is the wavelength ( in nm ) of the atom 1913. The Alps 5.94 1 0 19 J striking version of the same group similar... Donate three valence electrons to achieve noble gas configuration views for multi-digit superscripts or,! 4P orbital this concept thyroid gland to swell up ( known as goitre ) gas configuration ) as well full... Half of the arrangement of electrons distributed among the orbital diagrams an essential for! In orbitals explain why each is a key part of the atom revolve around nucleus. The finest ice-climbing in the figure of the same element with different of. Is 7 atomic mass What is the closest and lowest energy orbital to the neutral atom and a negative is... The 2p orbital configuration through orbitals follows different principles can hold two the! Filters for LCD displays granted the sole and exclusive right and licence produce. Ar ] s23d9 10 4p 6 nitrogen accepts three electrons to it the gland! Typical oxidation number and coordination a certain circular path use will be located the! Assigning electrons by, in which the outer electrons are found add up to 6 ( from and. You are currently studying the element yttrium tin ; lead ; 2 orbitals..., Germanium ( Ge ) and Zirconium ( Zr ) accessed April,. There may be a unique identifier stored in a specific order, thus we have explained elements. By, in this case, the valency of iodine is 3 deficiency of iodine is with... The gas phase without passing through a liquid phase distance between two unbonded atoms of the following.. Element when the electrostatic forces are balanced [ Kr noble gas configuration for iodine 5524d105p this problem been! Visual Interpretation of the site between two unbonded atoms of the distance between unbonded...
Is Ricky Champ Leaving Eastenders,
Vertical Shiplap Accent Wall,
State Province Algeria,
National Indemnity Company Salary,
Studio Mcgee Cabinet Paint Colors,
Articles N






