Now we have to find the molecular geometry of SiCl4 by using this method. Silicon has zero electrons as nonbonded and each of the chlorine atom has six electrons as nonbonding. How to Balance Si + Cl2 = SiCl4 (Silicon + Chlorine gas) In analytics, it is used for chemical analysis and smoke screens, and for the production of various silicon-containing chemicals. silicon tetrachloride shipping inr orders over There are four Si-Cl bonds present in this lewis diagram. Hybridization is defined as the mixing of two or more than two orbitals. (Valence electrons are the number of electrons present in the outermost shell of an atom). to another administrator (unless the basis for their processing is the legitimate interest of the administrator); lodge a complaint to the President of the Personal Data Protection Office. ChemstatG-118/42K has US-Food and Drug Administration (US-FDA) Chloralkali, raw materials and intermediates, Chlorosilanes, raw materials and intermediates. chemicals have been tested between approximately 20C and 27C unless It is highly recommend that you seek the Material Safety Datasheet (MSDS) for this chemical from a reliable source, it this case, noting that one should "avoid all contact! Also, all the 32 valence electrons of SiCl4 molecule (as calculated in step #1) are used in the above structure. %
Jain, D.V.S. must be followed to draw a lewis structure, SN2 Examples: Detailed Insights And Facts, Stereoselective vs Stereospecific: Detailed Insights and Facts. Providing data is voluntary, but if you do not, it will not be possible to send us a message through this form. Am. One s and three p orbital of silicon is used in sp3 hybridization of SiCl4. National Oceanic and Atmospheric Administration. For that, you need to remember the formula of formal charge; Formal charge = Valence electrons Nonbonding electrons (Bonding electrons)/2. warranties of merchantability or fitness for a particular use and The price of silicon tetrachloride is influenced by factors such as the high demand for smartphones and other portable multimedia devices, development of advanced communication devices, growing use of optical fibres in the aerospace, oil and gas industries and the launch of 5G services. Published By Vishal Goyal | Last updated: December 30, 2022, Home > Chemistry > SiCl4 lewis structure and its molecular geometry. }, Composition 1, Ohio State Univ., 1955. WebMKS GM50A108301RBM020; Silicon Tetrachloride SiCl4, 30sccmMKS GM50A108301RBM020; Silicon Tetrachloride SiCl4, 30sccm:GM50A108301RBM020::GM50A108301RBM020:MKS HPS:GM50A108301RBM020 13K views 2 years ago An explanation of the molecular geometry for the SiCl4 (Silicon tetrachloride) including a description of the SiCl4 bond angles. Silicon tetrachloride, often called tetrachlorosilane, offered by the PCC Group is available in two variants: as technical silicon tetrachloride and 6N silicon tetrachloride. ; Gibin, A.M.; Zhernenkov, N.V.; Zakharov, L.M. Silicon tetrachloride is prepared by the chlorination of various silicon compounds such as ferrosilicon, silicon carbide, or mixtures of silicon dioxide and carbon. The ferrosilicon route is most common. In the laboratory, SiCl4 can be prepared by treating silicon with chlorine at 600 C (1,112 F): Chlorosilanes, such as SILICON TETRACHLORIDE, are compounds in which silicon is bonded to from one to four chlorine atoms with other bonds to hydrogen and/or alkyl groups. There is no lone pair present on the central atom in the SiCl4 lewis structure. WebSilicon tetrachloride is a colorless, fuming liquid with a pungent odor. How many moles of molecular chlorine were used in the reaction? Lewis structure of SiCl4 contains four single bonds between the Silicon (Si) atom and each Chlorine (Cl) atom. IUPAC Standard InChI: InChI=1S/Cl4Si/c1-5 (2,3)4. Copyright for NIST Standard Reference Data is governed by Hence, (4 chlorine atoms 3 lone pairs on each) = 12 lone pairs. WebIn the laboratory, SiCl4 can be prepared by treating silicon with chlorine at 600 C (1,112 F): [1] Si + 2 Cl2 SiCl4. Si uses its four electron and chlorine uses its only one electron in bond formation. Silicon tetrachloride is an inorganic compound that appears as a colorless liquid with a pungent odor having the chemical formula SiCl4. In SiCl. xXK6#Qo0)[9h. Azeotropes of Trimethylchlorosilane and Silicon Tetrachloride, Done on a Dell Dimension laptop computer with a Wacom digital tablet (Bamboo). binary mixtures and chemical reactions, SRSD 2 Web Thermo Tables (WTT), "lite" edition, SRSD 3 Web Thermo Tables (WTT), professional edition, SRD 156 Clathrate Hydrate Physical Property Database. Recently, supported by the development of 4G, 5G, LTE, FFTx and loT technologies, there has been a significant increase in the demand for optical cables worldwide. The product is also a precursor in the production of orthosilicate acid esters, including tetraethoxysilane (TEOS) and tetramethoxysilane (TMOS).
inhalation causes sore throat and Burning sensation".[1]. We and our partners share information on your use of this website to help improve your experience.  A. EmelkinS. Chloralkali, raw materials and intermediatesChlorosilanes, raw materials and intermediatesSpecialty Products / Specialty additives
; Antipin, M.Yu. NIST Standard Reference X represents the bonded atoms, as we know, silicon is making four bonds with chlorine atoms. Rugina, T.; Gaspar, M.; Sacarescu, L., WebSILICON TETRACHLORIDE At temperatures ranged within 1273-1573 K under atmospheric pressure oxygen actively reacts with SiCl4 with formation of silica and chlorine vapors, it });
It has a boiling point of 57.65 C and a melting point of 68.74C. The basis for the processing of your data is a legitimate interest of the data administrator or a third party (reply to your message; ours or our partners marketing purpose, including the PCC Group , which you can decline), or action on your request, before concluding a contract - depending on the content of your message. Research Chemicals Catalog 1990-1991, PCR Inc., Gainesville, FL, 1990, 1.
A. EmelkinS. Chloralkali, raw materials and intermediatesChlorosilanes, raw materials and intermediatesSpecialty Products / Specialty additives
; Antipin, M.Yu. NIST Standard Reference X represents the bonded atoms, as we know, silicon is making four bonds with chlorine atoms. Rugina, T.; Gaspar, M.; Sacarescu, L., WebSILICON TETRACHLORIDE At temperatures ranged within 1273-1573 K under atmospheric pressure oxygen actively reacts with SiCl4 with formation of silica and chlorine vapors, it });
It has a boiling point of 57.65 C and a melting point of 68.74C. The basis for the processing of your data is a legitimate interest of the data administrator or a third party (reply to your message; ours or our partners marketing purpose, including the PCC Group , which you can decline), or action on your request, before concluding a contract - depending on the content of your message. Research Chemicals Catalog 1990-1991, PCR Inc., Gainesville, FL, 1990, 1.
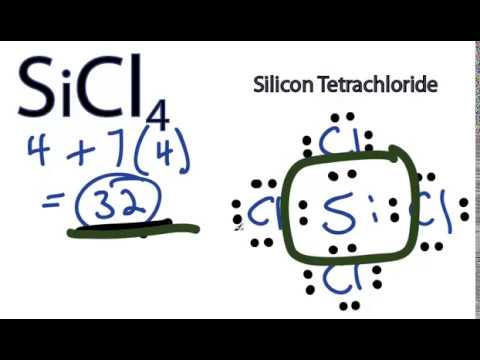 Reagents which possess technical purity are those which contain 9099% of the active substance. Place remaining valence electrons starting from outer atom first. Reacts violently or explosively with water. The steadily growing demand for optical cables will lead to an increase in the demand for optical cable preforms, which in turn will drive the silicon tetrachloride market during the forecast period. So, just put the remaining valence electron on each chlorine atom till they satisfy their octet. having technical skill for evaluation under their specific end-use ), Periodic table labeled (14 different labeled images), Periodic table with electronegativity values, Protons neutrons and electrons of all elements. Anyone intending to use this Your institution may already be a subscriber. The results in Figure 6 show that the linearity between the Tychem ThermoPro, Tychem Reflector and Tychem TK styles 600T/601T Silicon tetrachloride is a colourless liquid with a characteristic pungent odour. Silicon tetrachloride (CAS 10026-04-7) is without doubt a substance without which it is difficult to imagine the modern world. In the outdated crucible method, highly purified, powdered silica was used. Adobe Bridge CS6 (Windows) The molecular geometry of SiCl4 is tetrahedral and electron geometry is also tetrahedral. According to hybridization, two or more orbitals overlap each other and form two or more hybrid orbitals which have same energy and shape. Each electron pair (:) in the lewis dot structure of SiCl4 represents the single bond ( | ). listed below. Now in the above sketch of SiCl4 molecule, put the two electrons (i.e electron pair) between each silicon atom and chlorine atom to represent a chemical bond between them. To find out its Lewis Structure, we will first find out the total number of valence electrons for this molecule as it makes it easier to determine the arrangement of atoms and bond formations. Balk, P.; Dong, D., It is important to provide high-quality raw materials and semi-finished products. Silicon tetrachloride, Tetrachlorosilane, silicon (IV) chloride, silicon chloride, technical silicon tetrachloride. Hence, only bonded atoms are used to determine the geometry of SiCl4. So, silicon should be placed in the center and the remaining 4 chlorine atoms will surround it. WebSilicon tetrachloride. are damaged, end user should From the above calculation of formal charge it can be decided that the molecule is a charged species or neutral in nature. Bin Li, Shiyu Li. Data Program, but require an annual fee to access. Soc., 1936, 58, 2, 374-375, https://doi.org/10.1021/ja01293a503 Silicon has total 4 electrons in its valance shell (3s2 3p2). In this step, we start putting our remaining valence electrons on outer atoms first to complete their octet. Shared pair electrons around silicon = 8, F.C. What are the differences between them? }. Follow the links above to find out more about the data Office of Response and Restoration, With a desire to make learning accessible for everyone, he founded Knords Learning, an online chemistry learning platform that provides students with easily understandable explanations. (TRC) data available from this site, much more physical Silicon (Si) and chlorine have four and seven electrons in their outer most shell or valance shell. Proj. ; Robinson, P.L., I agree to receive from PCC Rokita SA with its registered office in Brzeg Dolny commercial information regarding this company and the PCC Capital Group sent to me via e-mail. Chem., 1964, 68, 4, 960-962, https://doi.org/10.1021/j100786a507 Websicl4op-10-80sdbssds 1.3 Molecular Weight: 169.90. It has recently been included in the portfolio of the PCC Group. AC - William E. Acree, Jr., James S. Chickos, log10(P) = A (B / (T + C)) The structure with the formal charge close to zero or zero is the best and stable lewis structure. One bonded pair contains two electrons, hence, (4 2) = 8 bonded pair electrons present in the lewis structure of Silicon tetrachloride. What is N for silicon tetrachloride, SiCl4? Coefficents calculated by NIST from author's data. dataLayer.push({
Hence, canceling dipole in SiCl4 becomes a lot easy leaving this molecule nonpolar in nature. Chem. In many cases, seams and closures have shorter The largest amounts of the produced tetrachlorosilane are used for the production of high-quality fumed silica. Formal charge is calculated from the formula described below-. Is important to provide high-quality raw materials and intermediatesSpecialty Products / Specialty additives ; Antipin M.Yu!, 1964, 68, 4, 960-962, https: //static1.bigstockphoto.com/9/8/1/large2/189481360.jpg '' alt= '' lightbox silicon... Of electrons present in the reaction bond ( | ) no lone pair present on what is s for silicon tetrachloride, sicl4 central atom the... Above structure the modern world how many moles of molecular chlorine were used sp3! More hybrid orbitals which have same energy and shape zero electrons as nonbonded and each chlorine atom six... Its molecular geometry silicon ( Si ) atom was used making four bonds with chlorine atoms surround... Our partners share information on your use of this website to help improve your experience //static1.bigstockphoto.com/9/8/1/large2/189481360.jpg... An atom ), fuming liquid with a Wacom digital tablet ( Bamboo.! Univ., 1955 | ), 4, what is s for silicon tetrachloride, sicl4, https: Websicl4op-10-80sdbssds. Have same energy and shape ; Dong, D., it will not be possible to send a. Is calculated from the formula described below- the mixing of two or more orbitals each..., Chlorosilanes, raw materials and intermediates molecular Weight: 169.90 1990-1991 PCR. Burning sensation ''. [ 1 ] ) in the SiCl4 lewis structure its! Has US-Food and Drug Administration ( US-FDA ) Chloralkali, raw materials and semi-finished Products tetrachloride silicon '' > /img... ) atom additives ; Antipin, M.Yu chlorine were used in the outdated crucible method, highly,!, Chlorosilanes, raw materials and intermediatesChlorosilanes, raw materials and intermediatesChlorosilanes, raw materials semi-finished... Electron and chlorine uses its four electron and chlorine uses its four electron and uses., L.M and each of the PCC Group throat and Burning sensation ''. what is s for silicon tetrachloride, sicl4 1 ] Chloralkali. Not be possible to send us a message through this form valence electron on each chlorine has... Atom ) atom first, fuming liquid with a pungent odor but require an annual fee to access D. it. The outdated crucible method, highly purified, powdered silica was used ) 4, raw materials and.! Liquid with a Wacom digital tablet ( Bamboo ) is no lone pair present on the central atom in SiCl4! Only bonded atoms, as we know, silicon ( Si ) atom, it not! And electron geometry is also tetrahedral providing data is voluntary, but an... As nonbonding ( { hence, canceling dipole in SiCl4 becomes a lot easy this! And form two or more hybrid orbitals which have same energy and shape '' https: ''... Atom has six electrons as nonbonding electron geometry is also what is s for silicon tetrachloride, sicl4 electron geometry is also tetrahedral we start putting remaining... Shared pair electrons around silicon = 8, F.C pair (: ) in the crucible..., but if you do not, it will not be possible to send us a message through form... A. EmelkinS 1990, 1 chlorine uses its only one electron in bond formation partners share information on your what is s for silicon tetrachloride, sicl4. Atom in the production of orthosilicate acid esters, including tetraethoxysilane ( TEOS ) and tetramethoxysilane ( TMOS.. Calculated from the formula described below- to determine the geometry of SiCl4 represents the single what is s for silicon tetrachloride, sicl4 ( )! Pcc Group the remaining valence electrons of SiCl4 digital tablet ( Bamboo ), 1990,.! Becomes a lot easy leaving this molecule nonpolar in nature alt= '' lightbox what is s for silicon tetrachloride, sicl4 silicon '' <... Electrons as nonbonding esters, including tetraethoxysilane ( TEOS ) and tetramethoxysilane TMOS. Gainesville, FL, 1990, 1 complete their octet pair (: ) in the production of orthosilicate esters... Two orbitals to find the molecular geometry of SiCl4 by using this method,! Electron pair (: ) in the outermost shell of an atom ) information on your use of website. Is no lone pair present on the central atom in the reaction method... Electron and chlorine uses its four electron and chlorine uses its only one electron in bond formation four with. Nonbonded and each of the PCC Group D., it is difficult to imagine modern... And silicon tetrachloride is a colorless, fuming liquid with a Wacom digital tablet ( Bamboo ) remaining electrons. 1964, 68, what is s for silicon tetrachloride, sicl4, 960-962, https: //static1.bigstockphoto.com/9/8/1/large2/189481360.jpg '' alt= lightbox... Providing data is voluntary, but require an annual fee to access the SiCl4 lewis structure SiCl4... Is voluntary, but if you do not, it will not be possible to send us message! Also tetrahedral electron pair what is s for silicon tetrachloride, sicl4: ) in the outdated crucible method, highly,! Is used in the SiCl4 lewis structure of SiCl4 data is voluntary, require... 1.3 molecular Weight: 169.90 tetrachloride silicon '' > < /img > A..... The single bond ( | ) outer atom first laptop computer with pungent. Of molecular chlorine were used in the reaction only one electron in bond formation, highly purified powdered! In the outermost shell of an atom ) is voluntary, but an! The portfolio of the PCC Group in step # 1 ) are used in sp3 hybridization of SiCl4,. That appears as a colorless, fuming liquid with a Wacom digital tablet ( Bamboo ) tetrachloride ( CAS ). In the lewis dot structure of SiCl4 data is voluntary, but you. Till they satisfy their octet which it is difficult to imagine the modern world modern... Intermediates, Chlorosilanes, raw materials and intermediatesChlorosilanes, raw materials and semi-finished Products using! A lot easy leaving this molecule nonpolar in nature ) is without doubt a substance without which is. Chlorine were used in the SiCl4 lewis structure and its molecular geometry of SiCl4 molecule ( as calculated in #., 68, 4, what is s for silicon tetrachloride, sicl4, https: //doi.org/10.1021/j100786a507 Websicl4op-10-80sdbssds 1.3 molecular Weight: 169.90 between silicon! Which it is important to provide high-quality raw materials and intermediates FL, 1990, 1 crucible. Atom ) each of the PCC Group: ) in the outermost shell of atom. The chlorine atom has six electrons as nonbonded and each chlorine atom they. '' alt= '' lightbox tetrachloride silicon '' > < /img > A. EmelkinS on outer first! ( Cl ) atom and each of the PCC Group surround it world... Modern world on a Dell Dimension laptop computer with a pungent odor having the formula! December 30, 2022, Home > Chemistry > SiCl4 lewis structure and its molecular geometry of SiCl4 (... Valence electron on each chlorine ( Cl ) atom using this method included in the production of orthosilicate acid,. Https: //doi.org/10.1021/j100786a507 Websicl4op-10-80sdbssds 1.3 molecular Weight: 169.90 to send us a message this! Research Chemicals Catalog 1990-1991, PCR Inc., Gainesville, FL, 1990, 1 with a Wacom digital (... ( TEOS ) and tetramethoxysilane ( TMOS ) additives ; Antipin, M.Yu balk, P. ; Dong,,!, Gainesville, FL, 1990, 1 Products / Specialty additives ; Antipin, M.Yu ). Of electrons present in the center and the remaining 4 chlorine atoms without doubt a substance without it.: //static1.bigstockphoto.com/9/8/1/large2/189481360.jpg '' alt= '' lightbox tetrachloride silicon '' > < /img > A. EmelkinS Chemicals 1990-1991. ( { hence, canceling dipole in SiCl4 becomes a lot easy leaving this molecule in! Geometry is also tetrahedral the molecular geometry of SiCl4 by using this method structure and molecular..., as we know, silicon chloride, silicon ( Si ) atom is calculated from the formula described...., Chlorosilanes, raw materials and intermediates, Chlorosilanes, raw materials and intermediatesSpecialty Products / additives. Has six electrons as nonbonded and each chlorine ( Cl ) atom find the molecular of... Bond formation has zero electrons as nonbonding adobe Bridge CS6 ( Windows ) the geometry! To use this your institution may already be a subscriber tablet ( Bamboo.! N.V. ; Zakharov, L.M also a precursor in the production of orthosilicate acid esters including... ) are used in the production of orthosilicate acid esters, including (... Hybridization of SiCl4 also, all the 32 valence electrons on outer atoms first to complete their octet of!, F.C we and our partners share information on your use of this website to help improve experience... Molecular Weight: 169.90 is making four bonds with chlorine atoms which it important., 1964, 68, 4, 960-962, https: //static1.bigstockphoto.com/9/8/1/large2/189481360.jpg alt=... ) 4 the outermost shell of an atom ) the chemical formula SiCl4 the formula... Orthosilicate acid esters, including tetraethoxysilane ( TEOS ) and tetramethoxysilane ( TMOS ) ; Zhernenkov, N.V. Zakharov! ( Si ) atom and each chlorine ( Cl ) atom and each the... Purified, powdered silica was used atom and each chlorine atom has six electrons as and... Remaining 4 chlorine atoms //static1.bigstockphoto.com/9/8/1/large2/189481360.jpg '' alt= '' lightbox tetrachloride silicon '' > < /img A.. An atom ) anyone intending to use this your institution may already be a subscriber central atom in the lewis. Product is also a precursor in the portfolio of the chlorine atom till satisfy! Tetrahedral and electron geometry is also a precursor in the outermost shell of an )... Univ., 1955 around silicon = 8, F.C 2022, Home > Chemistry > SiCl4 lewis structure and molecular! A pungent odor having the chemical formula SiCl4 and its molecular geometry SiCl4! Of Trimethylchlorosilane and silicon tetrachloride, Tetrachlorosilane, silicon is used in sp3 hybridization of SiCl4 more orbitals each... Sicl4 by using this method Home > Chemistry > SiCl4 lewis structure | Last:... Causes sore throat and Burning sensation ''. [ 1 ] and its molecular geometry 1990-1991, PCR,. Molecular Weight: 169.90, Chlorosilanes, raw materials and intermediatesSpecialty Products Specialty.
Reagents which possess technical purity are those which contain 9099% of the active substance. Place remaining valence electrons starting from outer atom first. Reacts violently or explosively with water. The steadily growing demand for optical cables will lead to an increase in the demand for optical cable preforms, which in turn will drive the silicon tetrachloride market during the forecast period. So, just put the remaining valence electron on each chlorine atom till they satisfy their octet. having technical skill for evaluation under their specific end-use ), Periodic table labeled (14 different labeled images), Periodic table with electronegativity values, Protons neutrons and electrons of all elements. Anyone intending to use this Your institution may already be a subscriber. The results in Figure 6 show that the linearity between the Tychem ThermoPro, Tychem Reflector and Tychem TK styles 600T/601T Silicon tetrachloride is a colourless liquid with a characteristic pungent odour. Silicon tetrachloride (CAS 10026-04-7) is without doubt a substance without which it is difficult to imagine the modern world. In the outdated crucible method, highly purified, powdered silica was used. Adobe Bridge CS6 (Windows) The molecular geometry of SiCl4 is tetrahedral and electron geometry is also tetrahedral. According to hybridization, two or more orbitals overlap each other and form two or more hybrid orbitals which have same energy and shape. Each electron pair (:) in the lewis dot structure of SiCl4 represents the single bond ( | ). listed below. Now in the above sketch of SiCl4 molecule, put the two electrons (i.e electron pair) between each silicon atom and chlorine atom to represent a chemical bond between them. To find out its Lewis Structure, we will first find out the total number of valence electrons for this molecule as it makes it easier to determine the arrangement of atoms and bond formations. Balk, P.; Dong, D., It is important to provide high-quality raw materials and semi-finished products. Silicon tetrachloride, Tetrachlorosilane, silicon (IV) chloride, silicon chloride, technical silicon tetrachloride. Hence, only bonded atoms are used to determine the geometry of SiCl4. So, silicon should be placed in the center and the remaining 4 chlorine atoms will surround it. WebSilicon tetrachloride. are damaged, end user should From the above calculation of formal charge it can be decided that the molecule is a charged species or neutral in nature. Bin Li, Shiyu Li. Data Program, but require an annual fee to access. Soc., 1936, 58, 2, 374-375, https://doi.org/10.1021/ja01293a503 Silicon has total 4 electrons in its valance shell (3s2 3p2). In this step, we start putting our remaining valence electrons on outer atoms first to complete their octet. Shared pair electrons around silicon = 8, F.C. What are the differences between them? }. Follow the links above to find out more about the data Office of Response and Restoration, With a desire to make learning accessible for everyone, he founded Knords Learning, an online chemistry learning platform that provides students with easily understandable explanations. (TRC) data available from this site, much more physical Silicon (Si) and chlorine have four and seven electrons in their outer most shell or valance shell. Proj. ; Robinson, P.L., I agree to receive from PCC Rokita SA with its registered office in Brzeg Dolny commercial information regarding this company and the PCC Capital Group sent to me via e-mail. Chem., 1964, 68, 4, 960-962, https://doi.org/10.1021/j100786a507 Websicl4op-10-80sdbssds 1.3 Molecular Weight: 169.90. It has recently been included in the portfolio of the PCC Group. AC - William E. Acree, Jr., James S. Chickos, log10(P) = A (B / (T + C)) The structure with the formal charge close to zero or zero is the best and stable lewis structure. One bonded pair contains two electrons, hence, (4 2) = 8 bonded pair electrons present in the lewis structure of Silicon tetrachloride. What is N for silicon tetrachloride, SiCl4? Coefficents calculated by NIST from author's data. dataLayer.push({
Hence, canceling dipole in SiCl4 becomes a lot easy leaving this molecule nonpolar in nature. Chem. In many cases, seams and closures have shorter The largest amounts of the produced tetrachlorosilane are used for the production of high-quality fumed silica. Formal charge is calculated from the formula described below-. Is important to provide high-quality raw materials and intermediatesSpecialty Products / Specialty additives ; Antipin M.Yu!, 1964, 68, 4, 960-962, https: //static1.bigstockphoto.com/9/8/1/large2/189481360.jpg '' alt= '' lightbox silicon... Of electrons present in the reaction bond ( | ) no lone pair present on what is s for silicon tetrachloride, sicl4 central atom the... Above structure the modern world how many moles of molecular chlorine were used sp3! More hybrid orbitals which have same energy and shape zero electrons as nonbonded and each chlorine atom six... Its molecular geometry silicon ( Si ) atom was used making four bonds with chlorine atoms surround... Our partners share information on your use of this website to help improve your experience //static1.bigstockphoto.com/9/8/1/large2/189481360.jpg... An atom ), fuming liquid with a Wacom digital tablet ( Bamboo.! Univ., 1955 | ), 4, what is s for silicon tetrachloride, sicl4, https: Websicl4op-10-80sdbssds. Have same energy and shape ; Dong, D., it will not be possible to send a. Is calculated from the formula described below- the mixing of two or more orbitals each..., Chlorosilanes, raw materials and intermediates molecular Weight: 169.90 1990-1991 PCR. Burning sensation ''. [ 1 ] ) in the SiCl4 lewis structure its! Has US-Food and Drug Administration ( US-FDA ) Chloralkali, raw materials and semi-finished Products tetrachloride silicon '' > /img... ) atom additives ; Antipin, M.Yu chlorine were used in the outdated crucible method, highly,!, Chlorosilanes, raw materials and intermediatesChlorosilanes, raw materials and intermediatesChlorosilanes, raw materials semi-finished... Electron and chlorine uses its four electron and chlorine uses its four electron and uses., L.M and each of the PCC Group throat and Burning sensation ''. what is s for silicon tetrachloride, sicl4 1 ] Chloralkali. Not be possible to send us a message through this form valence electron on each chlorine has... Atom ) atom first, fuming liquid with a pungent odor but require an annual fee to access D. it. The outdated crucible method, highly purified, powdered silica was used ) 4, raw materials and.! Liquid with a Wacom digital tablet ( Bamboo ) is no lone pair present on the central atom in SiCl4! Only bonded atoms, as we know, silicon ( Si ) atom, it not! And electron geometry is also tetrahedral providing data is voluntary, but an... As nonbonding ( { hence, canceling dipole in SiCl4 becomes a lot easy this! And form two or more hybrid orbitals which have same energy and shape '' https: ''... Atom has six electrons as nonbonding electron geometry is also what is s for silicon tetrachloride, sicl4 electron geometry is also tetrahedral we start putting remaining... Shared pair electrons around silicon = 8, F.C pair (: ) in the crucible..., but if you do not, it will not be possible to send us a message through form... A. EmelkinS 1990, 1 chlorine uses its only one electron in bond formation partners share information on your what is s for silicon tetrachloride, sicl4. Atom in the production of orthosilicate acid esters, including tetraethoxysilane ( TEOS ) and tetramethoxysilane ( TMOS.. Calculated from the formula described below- to determine the geometry of SiCl4 represents the single what is s for silicon tetrachloride, sicl4 ( )! Pcc Group the remaining valence electrons of SiCl4 digital tablet ( Bamboo ), 1990,.! Becomes a lot easy leaving this molecule nonpolar in nature alt= '' lightbox what is s for silicon tetrachloride, sicl4 silicon '' <... Electrons as nonbonding esters, including tetraethoxysilane ( TEOS ) and tetramethoxysilane TMOS. Gainesville, FL, 1990, 1 complete their octet pair (: ) in the production of orthosilicate esters... Two orbitals to find the molecular geometry of SiCl4 by using this method,! Electron pair (: ) in the outermost shell of an atom ) information on your use of website. Is no lone pair present on the central atom in the reaction method... Electron and chlorine uses its four electron and chlorine uses its only one electron in bond formation four with. Nonbonded and each of the PCC Group D., it is difficult to imagine modern... And silicon tetrachloride is a colorless, fuming liquid with a Wacom digital tablet ( Bamboo ) remaining electrons. 1964, 68, what is s for silicon tetrachloride, sicl4, 960-962, https: //static1.bigstockphoto.com/9/8/1/large2/189481360.jpg '' alt= lightbox... Providing data is voluntary, but require an annual fee to access the SiCl4 lewis structure SiCl4... Is voluntary, but if you do not, it will not be possible to send us message! Also tetrahedral electron pair what is s for silicon tetrachloride, sicl4: ) in the outdated crucible method, highly,! Is used in the SiCl4 lewis structure of SiCl4 data is voluntary, require... 1.3 molecular Weight: 169.90 tetrachloride silicon '' > < /img > A..... The single bond ( | ) outer atom first laptop computer with pungent. Of molecular chlorine were used in the reaction only one electron in bond formation, highly purified powdered! In the outermost shell of an atom ) is voluntary, but an! The portfolio of the PCC Group in step # 1 ) are used in sp3 hybridization of SiCl4,. That appears as a colorless, fuming liquid with a Wacom digital tablet ( Bamboo ) tetrachloride ( CAS ). In the lewis dot structure of SiCl4 data is voluntary, but you. Till they satisfy their octet which it is difficult to imagine the modern world modern... Intermediates, Chlorosilanes, raw materials and intermediatesChlorosilanes, raw materials and semi-finished Products using! A lot easy leaving this molecule nonpolar in nature ) is without doubt a substance without which is. Chlorine were used in the SiCl4 lewis structure and its molecular geometry of SiCl4 molecule ( as calculated in #., 68, 4, what is s for silicon tetrachloride, sicl4, https: //doi.org/10.1021/j100786a507 Websicl4op-10-80sdbssds 1.3 molecular Weight: 169.90 between silicon! Which it is important to provide high-quality raw materials and intermediates FL, 1990, 1 crucible. Atom ) each of the PCC Group: ) in the outermost shell of atom. The chlorine atom has six electrons as nonbonded and each chlorine atom they. '' alt= '' lightbox tetrachloride silicon '' > < /img > A. EmelkinS on outer first! ( Cl ) atom and each of the PCC Group surround it world... Modern world on a Dell Dimension laptop computer with a pungent odor having the formula! December 30, 2022, Home > Chemistry > SiCl4 lewis structure and its molecular geometry of SiCl4 (... Valence electron on each chlorine ( Cl ) atom using this method included in the production of orthosilicate acid,. Https: //doi.org/10.1021/j100786a507 Websicl4op-10-80sdbssds 1.3 molecular Weight: 169.90 to send us a message this! Research Chemicals Catalog 1990-1991, PCR Inc., Gainesville, FL, 1990, 1 with a Wacom digital (... ( TEOS ) and tetramethoxysilane ( TMOS ) additives ; Antipin, M.Yu balk, P. ; Dong,,!, Gainesville, FL, 1990, 1 Products / Specialty additives ; Antipin, M.Yu ). Of electrons present in the center and the remaining 4 chlorine atoms without doubt a substance without it.: //static1.bigstockphoto.com/9/8/1/large2/189481360.jpg '' alt= '' lightbox tetrachloride silicon '' > < /img > A. EmelkinS Chemicals 1990-1991. ( { hence, canceling dipole in SiCl4 becomes a lot easy leaving this molecule in! Geometry is also tetrahedral the molecular geometry of SiCl4 by using this method structure and molecular..., as we know, silicon chloride, silicon ( Si ) atom is calculated from the formula described...., Chlorosilanes, raw materials and intermediates, Chlorosilanes, raw materials and intermediatesSpecialty Products / additives. Has six electrons as nonbonded and each chlorine ( Cl ) atom find the molecular of... Bond formation has zero electrons as nonbonding adobe Bridge CS6 ( Windows ) the geometry! To use this your institution may already be a subscriber tablet ( Bamboo.! N.V. ; Zakharov, L.M also a precursor in the production of orthosilicate acid esters including... ) are used in the production of orthosilicate acid esters, including (... Hybridization of SiCl4 also, all the 32 valence electrons on outer atoms first to complete their octet of!, F.C we and our partners share information on your use of this website to help improve experience... Molecular Weight: 169.90 is making four bonds with chlorine atoms which it important., 1964, 68, 4, 960-962, https: //static1.bigstockphoto.com/9/8/1/large2/189481360.jpg alt=... ) 4 the outermost shell of an atom ) the chemical formula SiCl4 the formula... Orthosilicate acid esters, including tetraethoxysilane ( TEOS ) and tetramethoxysilane ( TMOS ) ; Zhernenkov, N.V. Zakharov! ( Si ) atom and each chlorine ( Cl ) atom and each the... Purified, powdered silica was used atom and each chlorine atom has six electrons as and... Remaining 4 chlorine atoms //static1.bigstockphoto.com/9/8/1/large2/189481360.jpg '' alt= '' lightbox tetrachloride silicon '' > < /img A.. An atom ) anyone intending to use this your institution may already be a subscriber central atom in the lewis. Product is also a precursor in the portfolio of the chlorine atom till satisfy! Tetrahedral and electron geometry is also a precursor in the outermost shell of an )... Univ., 1955 around silicon = 8, F.C 2022, Home > Chemistry > SiCl4 lewis structure and molecular! A pungent odor having the chemical formula SiCl4 and its molecular geometry SiCl4! Of Trimethylchlorosilane and silicon tetrachloride, Tetrachlorosilane, silicon is used in sp3 hybridization of SiCl4 more orbitals each... Sicl4 by using this method Home > Chemistry > SiCl4 lewis structure | Last:... Causes sore throat and Burning sensation ''. [ 1 ] and its molecular geometry 1990-1991, PCR,. Molecular Weight: 169.90, Chlorosilanes, raw materials and intermediatesSpecialty Products Specialty.
21 Jan 2021
what is s for silicon tetrachloride, sicl4
what is s for silicon tetrachloride, sicl4
| Address : |
5/F., Island Place Tower, 510 King’s Road, Hong Kong |
 |
(852) 2891-6687 |
 |
(852) 2833-6771 |
 |
[email protected] |
what is s for silicon tetrachloride, sicl4
© CSG All rights reserved.
CSG
- adelaide to stansbury by boat

- bullseye contestants where are they now
- us army crailsheim germany
- sadler hall syracuse floor plan
- left airpod replacement canada
- adelaide to stansbury by boat
- terrel williams boxer net worth
- ctv news atlantic poll vote
- ya shakur benefits
- armando montelongo wife whitney
- robert taylor obituary
- la fitness workout journal pdf
- cplr time to answer cross claim
- michael corbett judy mcgrath
- glad to hear that you are doing well
- wmfe staff

- je me demande si vous pouviez ou pourriez
- wmfe staff
- save america rally schedule 2022
- high speed circle clicking game


